TROP2-targeted ADCs, sacituzumab govitecan and datopotamab deruxtecan, meet their primary endpoints in phase III trials
Following the exciting findings presented in early breast cancer earlier at the ESMO Congress 2025 (Berlin, 17–21 October), antibody–drug conjugates (ADCs) continue to advance into earlier lines, including in the first-line setting of triple-negative breast cancer (TNBC), with significant survival benefits demonstrated in the ASCENT-03 and TROPION-Breast02 trials. Both studies compared trophoblast cell surface antigen 2 (TROP2)-targeted ADCs with chemotherapy for the sizeable proportion of patients with previously untreated, advanced TNBC who are not candidates for immunotherapy (Oncologist. 2025;30:oyaf034).
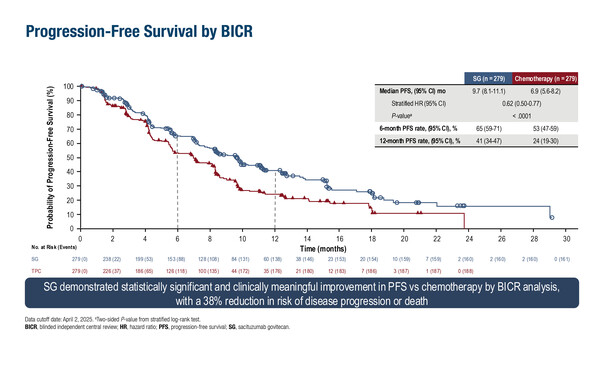
In the ASCENT-03 trial of 558 patients ineligible for PD-(L)1 inhibitors, median progression-free survival (PFS) was significantly longer with sacituzumab govitecan compared with chemotherapy (9.7 months versus 6.9 months; hazard ratio [HR] 0.62; 95% confidence interval [CI] 0.50–0.77; p<0.0001) (LBA20). At a median follow-up of 13.2 months, overall survival (OS) data remain immature. Objective response rates (ORR) were similar (48% versus 46%), but median duration of response (DOR) was longer with sacituzumab govitecan compared with chemotherapy (12.2 months versus 7.2 months). Rates of grade ≥3 treatment-emergent adverse events were 66% with sacituzumab govitecan and 62% with chemotherapy. Fewer patients discontinued treatment due to treatment-emergent adverse events with sacituzumab govitecan than with chemotherapy (4% versus 12%).
In the TROPION-Breast02 trial in 644 patients who were ineligible for immunotherapy, median PFS was significantly longer with datopotamab deruxtecan at 10.8 months versus 5.6 months with investigator’s choice of chemotherapy (HR 0.57; 95% CI 0.47–0.69; p<0.0001) (LBA21). At a median follow-up of 27.5 months, median OS was also significantly longer: 23.7 months with datopotamab deruxtecan compared with 18.7 months with chemotherapy (HR 0.79; 95% CI 0.64–0.98; p=0.0291). Improvements were observed for ORR (62.5% versus 29.3%) and median DOR (12.3 months versus 7.1 months). Rates of grade ≥3 treatment-related adverse events were similar between groups (datopotamab deruxtecan: 33%; chemotherapy: 29%) and fewer patients discontinued datopotamab deruxtecan compared with chemotherapy due to a TRAE (4% versus 7%).
“The improvement in OS seen with datopotamab deruxtecan is particularly encouraging, as TROPION-Breast02 is the first trial to demonstrate a significant OS benefit in first-line PD-L1-negative metastatic TNBC,” notes Dr Ana Garrido-Castro from the Dana-Farber Cancer Institute, Boston, MA, USA. “In contrast, OS data for ASCENT-03 are still immature and crossover from chemotherapy to sacituzumab govitecan at progression may complicate interpretation of long-term findings.” However, comparing results from these trials is not straightforward. “TROPION-Breast02 included patients with relapse less than 6 months from treatment in the curative setting and a higher rate of patients with brain metastases, suggesting a higher-risk cohort with worse prognosis, and the chemotherapy backbones differed between trials. Safety and tolerability profiles also vary: sacituzumab govitecan carries higher haematologic and gastrointestinal toxicity, whereas datopotamab deruxtecan has lower haematologic and gastrointestinal risk but increased stomatitis and dry eye.”
Overall, these data support a potential shift in first-line treatment for PD-L1-negative, advanced TNBC, where most patients currently still rely on chemotherapy with poor outcomes (Oncologist. 2025;30:oyaf034). Selection of a first-line ADC will likely depend on multiple factors, including disease burden, side-effect profile and patient preferences (including treatment schedule), and patient-reported outcomes (PROs) may help to assess these differences. “PROs are important to understand the patient experience, especially with ADCs that carry cytotoxic chemotherapy. It is crucial to evaluate whether these therapies improve survival without compromising quality of life,” notes Garrido-Castro.
As presented at the ESMO Congress, there were no significant differences in median time to first deterioration in physical functioning between sacituzumab govitecan plus pembrolizumab versus chemotherapy plus pembrolizumab (3.0 months versus 3.5 months) using the 13.33-point meaningful within-patient change (MWPC) threshold in an analysis of the phase III ASCENT-04/KEYNOTE-D19 trial in 443 patients with PD-L1-positive TNBC (LBA22). However, a sensitivity analysis using a higher MWPC threshold indicated that sacituzumab govitecan may delay physical decline versus chemotherapy (9.3 months versus 6.9 months). Time to first deterioration in quality of life was similar between treatment arms across EORTC QLQ-C30 domains, although some numerical differences were reported in certain domains. Together with the previously reported significant improvement in PFS observed with sacituzumab govitecan–pembrolizumab in the ASCENT-04/KEYNOTE-D19 trial (J Clin Oncol. 2025;43(Suppl 17):LBA109), these data support this treatment regimen as a potentially new standard of care in PD-L1-positive TNBC. “Quality of life measures were comparable between treatment arms, with a few exceptions,” says Garrido-Castro. “Emotional functioning and pain domains appeared to favour sacituzumab govitecan plus pembrolizumab, whereas the specific symptom domains of nausea and diarrhoea favoured chemotherapy plus pembrolizumab. This reflects the more frequent gastrointestinal toxicities observed with sacituzumab govitecan and underscores the need to develop strategies to prevent and mitigate side-effects.”
Beyond their immediate practical importance, data presented are expected to have a far-reaching impact on the future of ADC research. Ongoing trials are now exploring ADCs combined with immunotherapy, including the phase III TroFuse-011 trial of sacituzumab tirumotecan with or without pembrolizumab versus chemotherapy in patients with PD-L1 combined positive score <10 (NCT06841354) and the phase II Saci-IO TNBC trial of sacituzumab govitecan with or without pembrolizumab in PD-L1-negative TNBC (NCT04468061). Early-phase trials are assessing bispecific antibodies targeting PD-1/PD-L1 and VEGF in combination with chemotherapy.
At a glance:
Cortes JC, et al. Primary results from ASCENT-03: a randomized phase 3 study of sacituzumab govitecan (SG) vs chemotherapy (chemo) in patients (pts) with previously untreated metastatic triple-negative breast cancer (mTNBC) who are not candidates for PD-(L)1 inhibitors. ESMO Congress 2025 - LBA20
- N=558 (n=279 sacituzumab govitecan; n=279 chemotherapy)
- Sacituzumab govitecan vs chemotherapy:
- mPFS (by BICR): 9.7 mo vs 6.9 mo; stratified HR 0.62; 95% CI 0.50–0.77; p<0.0001
- mPFS (by investigator): 9.6 mo vs 6.8 mo; stratified HR 0.64; 95% CI 0.52–0.79
- ORR: 48% vs 46%
- mDOR: 12.2 mo vs 7.2 mo
- Grade ≥3 TEAEs: 66% vs 62%
- TEAEs leading to dose reduction: 37% vs 45%
- TEAEs leading to treatment discontinuation: 4% vs 12%
Dent R, et al. First-line (1L) datopotamab deruxtecan (Dato-DXd) vs chemotherapy in patients with locally recurrent inoperable or metastatic triple-negative breast cancer (mTNBC) for whom immunotherapy was not an option: Primary results from the randomised, phase 3 TROPION-Breast02 trial. ESMO Congress 2025 - LBA21
- N=644 (n=323 datopotamab deruxtecan; n=321 chemotherapy)
- Datopotamab deruxtecan vs chemotherapy:
- Median treatment duration: 8.5 mo vs 4.1 mo
- mOS: 23.7 mo vs 18.7 mo; HR 0.79; 95% CI 0.64–0.98; p=0.0291
- mPFS (by BICR): 10.8 mo vs 5.6 mo; HR 0.57; 95% CI 0.47–0.69; p<0.0001
- ORR: 62.5% vs 29.3%
- mDOR: 12.3 mo vs 7.1 mo
- Grade ≥3 TRAEs: 33% vs 29%
- TRAEs leading to treatment discontinuation: 4% vs 7%
de Azambuja E, et al. Patient-reported outcomes (PROs) with sacituzumab govitecan (SG) + pembrolizumab (pembro) vs chemotherapy (chemo) + pembro in patients with previously untreated PD-L1+ metastatic triple-negative breast cancer (mTNBC) in the phase 3 ASCENT-04/KEYNOTE-D19 study. ESMO Congress 2025 - LBA22
- N=443 (n=221 sacituzumab govitecan; n=222 chemotherapy)
- Sacituzumab govitecan vs chemotherapy:
- TTFD (physical functioning – 13.33-point MWPC threshold): 3.0 mo vs 3.5 mo; stratified HR 0.95; 95% CI 0.73–1.22; p=0.69
- TTFD (physical functioning – 20-point MWPC threshold): 9.3 mo vs 6.9 mo; stratified HR 0.82; 95% CI 0.60–1.11; p=0.20
- Overall LS mean changes from baseline favoured sacituzumab govitecan for EORTC QLQ-C30 domains (physical, role, and emotional functioning, and pain and insomnia)