Follow-up data at 7 and 5 years from monarchE and NATALEE provide reassurance of the sustained benefits of adjuvant CDK4/6 inhibitors in improving survival and reducing relapse
Previous results from the phase III monarchE (Lancet Oncol. 2023;24:77–90) and NATALEE trials (N Engl J Med. 2024;390:1080–1091) demonstrated the significant benefit of adjuvant CDK4/6 inhibitors in prolonging invasive disease-free survival (IDFS) in patients with high-risk hormone receptor-positive, HER2-negative early breast cancer. Building on these findings, longer follow-up results from these trials presented at the ESMO Congress 2025 (Berlin, 17–21 October) reinforce the role of CDK4/6 inhibitors in the adjuvant setting. “For high-risk patients, defined not only by nodal status or stage, but also by aggressive tumour features such as high grade or a high Oncotype DX score, there is a clear and sustained benefit to adding a CDK4/6 inhibitor – whether ribociclib or abemaciclib – to endocrine therapy,” says Dr Angela DeMichele from the University of Pennsylvania, Philadelphia, PA, USA.
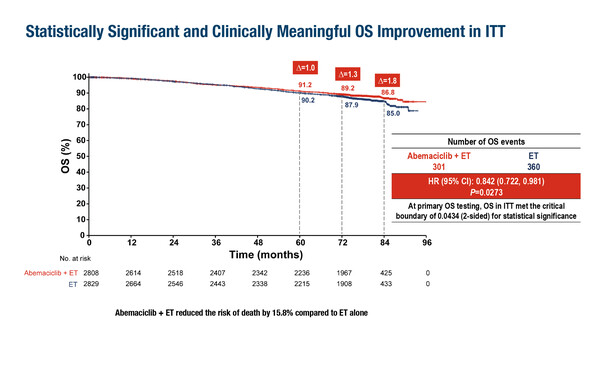
In the primary overall survival (OS) analysis of the monarchE trial, abemaciclib plus endocrine therapy reduced the risk of death by 15.8% compared with endocrine therapy alone (hazard ratio [HR] 0.842; p=0.0273) in 5,637 patients at a median follow-up of 76 months (LBA13, key results in the box below). At the 7-year landmark analysis, a 26.6% reduction in the risk of IDFS (nominal p<0.0001) and a 25.4% reduction in the risk of distant relapse-free survival (DRFS; nominal p<0.0001) were also observed with the addition of abemaciclib. “This is a disease with a long natural history, so we require lengthy follow-up to establish survival benefits. Having 7-year data helps us to feel better informed about outcomes for these patients,” comments DeMichele. “An OS benefit has now emerged with abemaciclib; however, the absolute difference in OS between treatment arms is modest and the larger benefit appears to be in preventing relapse. This is good news for patients in whom the priority is to avoid metastatic disease, but the discrepancy between relapse-free survival and OS requires longer follow-up and raises important questions about the impact of receiving adjuvant abemaciclib on post-relapse outcomes.”
The NATALEE trial, also discussed at the Congress, is currently 2 years behind monarchE in terms of data analysis, but it seems to be on track to show similar overall benefits. In 5,101 patients, the 5-year risk of IDFS was reduced by 28.4% with adjuvant ribociclib plus a non-steroidal aromatase inhibitor (NSAI) compared with an NSAI alone (nominal p<0.0001) (LBA14, key results in the box below). Additionally, 5-year distant disease-free survival and DRFS rates were reduced by 29.1% and 30.1%, respectively. Over a median follow-up of 56.5 months, a positive trend toward improved OS was observed with adjuvant ribociclib plus NSAI (HR 0.800; nominal p=0.026). Commenting on these findings, DeMichele says, “We anticipate further PFS and potentially OS benefits to emerge with longer follow-up.” She adds, “Findings from the NATALEE trial broaden the potential role of CDK4/6 inhibitors in early breast cancer, extending their use to patients with node-negative disease who showed statistically significant reductions in relapse in the 5-year analysis. Together, these data could help expand the availability of CDK4/6 inhibitors to broader geographical areas where their uptake is still limited and access depends on demonstrating OS benefit or substantial relapse reduction.”
Looking to the future, DeMichele highlights the emerging area of cancer cell dormancy and the potential role for CDK4/6 inhibitors in this context. “Adjuvant therapy targets actively dividing cells that may have left the breast tissue, but there is another population, known as dormant cells, that hibernate in the body before reactivating and forming metastases,” she explains. “These cells are often insensitive to standard therapies, but we are beginning to explore whether CDK4/6 inhibitors can target them. This could represent an additional mechanism by which these agents benefit patients.” She concludes, “If we can eliminate both dividing and dormant cells, we may ultimately prevent relapse in all patients. Being able to identify and monitor for these cells would also provide enormous reassurance to patients, confirming that their risk of recurrence has been minimised. It is a very exciting time, and this area will continue to evolve rapidly.”
At a glance:
Johnston SRD, et al. monarchE: Primary overall survival (OS) results of adjuvant abemaciclib + endocrine therapy (ET) for HR+, HER2-, high-risk early breast cancer (EBC). ESMO Congress 2025 - LBA13
- N=5,637 (abemaciclib + ET: n=2,808; ET: n=2,829)
- Abemaciclib + ET vs ET:
- OS: 86.8% vs 85.0%; HR 0.842; 95% CI 0.722–0.981; p=0.0273
- Pts living with metastatic disease: 14.3% vs 19.9%
- IDFS: 77.4% vs 70.9% ; HR 0.734; 95% CI 0.657–0.820; nominal p<0.0001
- DRFS: 80.0% vs 74.9%; HR 0.746; 95% CI 0.662–0.840; nominal p<0.0001
- AEs leading to death (on-therapy): 0.5% vs 0.4%
- AEs leading to death (post-discontinuation): 1.6% vs 1.1%
Crown JP, et al. Adjuvant ribociclib (RIB) plus nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+/HER2− early breast cancer (EBC): NATALEE 5-year outcomes. ESMO Congress 2025 - LBA14
- N=5,101 (ribociclib + NSAI: n=2,549; NSAI: n=2,552)
- Ribociclib + NSAI vs NSAI:
- OS: 94.1% vs 92.5%; HR 0.800; 95% CI 0.637–1.003; nominal p-value (1-sided)=0.026
- IDFS: 85.5% vs 81.0%; HR 0.716; 95% CI 0.618–0.829; nominal p-value (1-sided)<0.0001
- DDFS: 86.8% vs 82.5%; HR 0.709; 95% CI 0.608–0.827
- DRFS: 88.4% vs 84.4%; HR 0.699; 95% CI 0.594–0.824