Longer follow-up from two studies supports neoadjuvant over adjuvant immune checkpoint inhibition in patients with resectable melanoma and clinically or radiologically detectable metastasis, plus useful insights gained from biomarker analyses will help refine treatment strategies
Updated analyses from the phase III NADINA trial and the phase II SWOG S1801 trial confirm the sustained benefits of neoadjuvant immune checkpoint inhibition (ICI) in patients with resectable stage III or IV melanoma, at 2 and 3 years of follow-up, respectively. Data were presented at the ESMO Congress 2025 (Berlin, 17–21 October) and, according to Prof. Teresa Amaral from ICANS, Strasbourg, France, “They are particularly important as there is still some reluctance and hurdles to approve and adopt the neoadjuvant approach in this setting in many countries.”
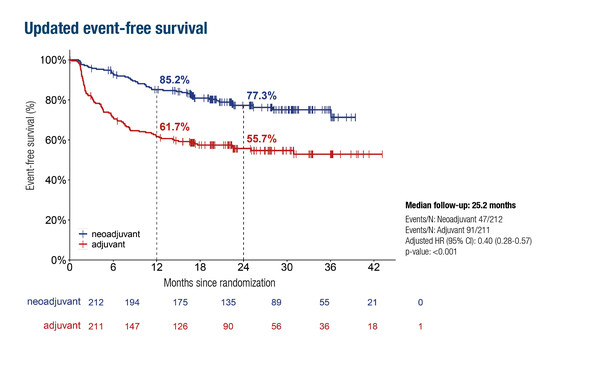
The phase III NADINA trial has previously shown that neoadjuvant ipilimumab plus nivolumab followed by surgery and response-driven adjuvant therapy increased 12-month event-free survival (EFS) rates compared with surgery followed by adjuvant nivolumab (N Engl J Med. 2024;391:1696–1708). Updated findings (n=423) show 22% higher estimated 2-year EFS rates of 77.3% in the neoadjuvant arm versus 55.7% in the adjuvant arm (hazard ratio [HR] 0.40; 95% confidence interval [CI] 0.28–0.57; p<0.001) (LBA57, key results in the box below).
In the primary analysis of the phase II SWOG S1801 trial, EFS after a median follow-up of ~15 months was significantly higher among those who received pembrolizumab both before and after surgery than among those who received adjuvant pembrolizumab alone (N Engl J Med. 2023;388:813–823). In the new analysis (n=345), 3-year EFS rates were 68% in the neoadjuvant arm and 56% in the adjuvant arm (minimum follow-up of 3 years: HR for EFS 0.67; 95% CI 0.42–0.94) (Abstract 1601O, key results in the box below). OS data appear to be favourable, but mature data are needed.
Amaral explains: “The main message is clearly that neoadjuvant immunotherapy is better than the adjuvant approach alone in this setting (resectable melanoma with pathologically proven, clinically or radiologically detectable metastasis), and we need to continue advocating for increased availability for patients with neoadjuvant suitability criteria. We still do not have the answer for what constitutes the optimal neoadjuvant strategy, including how many cycles patients should receive and whether ipilimumab plus nivolumab is needed in all patients. There is no ‘one size fits all’. Further biomarker analyses will help define the most appropriate neoadjuvant approach for each patient.”
Biomarker analysis is, in fact, highly valuable to determine which patients may benefit most from upfront combined ICI over anti-PD-1 therapy alone. In Berlin, a Mini Oral presentation described the creation of a promising predictive model, which involved testing combinations of clinical features, RNA sequencing data and DNA sequencing data to help identify patients resistant to neoadjuvant anti-PD-1 therapy (Abstract 117MO, key results in the box below). The consensus ‘NeoPredict’ model includes 4 clinical features, 7 RNA transcriptomic expression signatures and 2 DNA features, and demonstrated good performance for predicting non-major pathological response. NeoPredict will now need to be validated prospectively. “To offer the best treatment possible to all patients, we must not forget the need to make biomarkers and their determination available broadly,” Amaral concludes.
At a glance:
Lucas MW, et al. Two-year clinical update and first biomarker analyses of the phase III NADINA trial comparing neoadjuvant nivolumab plus ipilimumab versus adjuvant nivolumab in resectable stage III melanoma. ESMO Congress 2025 - LBA57
- Neoadjuvant nivolumab + ipilimumab vs adjuvant nivolumab (n=423)
- 2-y EFS: 77.3% vs 55.7% (HR 0.40; 95% CI 0.28–0.57; p<0.001)
- 2-y DMFS: 82.8% vs 63.9% (HR 0.43; 95% CI 0.29–0.64; p<0.001)
- Grade ≥3 systemic TRAEs: 31.1% vs 15.9%
Patel SP, et al. 3-year survival with neoadjuvant-adjuvant pembrolizumab from SWOG S1801. ESMO Congress 2025 - Abstract 1601O
- Neoadjuvant-adjuvant pembrolizumab vs adjuvant pembrolizumab (n=345)
- 3-y EFS: 68% vs 56%
- 3-y RFS: 80% vs 60%
- 3-y OS: 84% vs 73%
- Grade 3–4 TRAEs: 21% vs 18%
Pires da Silva I, et al. Clinical and multi-omics predictive model of resistance to neoadjuvant anti-PD1 (PD1) in melanoma. ESMO Congress 2025 - Abstract 117MO
- Consensus model: 4 clinical features, 7 RNA transcriptomic expression signatures and 2 DNA features
- AUC: 0.84 (95% CI 0.77–0.91) for predicting non-MPR