Tarlatamab as first-line therapy and ceralasertib plus durvalumab as maintenance therapy have demonstrated potential efficacy and manageable tolerability
The current standard of care for extensive-stage small cell lung cancer (ES-SCLC), first-line platinum-based chemotherapy combined with immunotherapy, is associated with only a modest median overall survival benefit of 2 to 2.5 months over chemotherapy alone. Addressing an ongoing unmet need to improve initial treatment outcomes and long-term disease control, early-phase clinical trials presented at the ESMO Congress 2025 (Berlin, 17–21 October) show the potential of regimens incorporating novel agents such as tarlatamab and ceralasertib.
The phase Ib DeLLphi-303 study reported an objective response rate (ORR) of 71% and median progression-free survival (mPFS) of 10.3 months in 96 patients with ES-SCLC who received first-line tarlatamab in combination with platinum–etoposide and a PD-L1 inhibitor, followed by tarlatamab plus maintenance PD-L1 inhibitor therapy (Abstract 2757O, key results in the box below). Targeting a surface marker expressed on >80% of small cell lung cancer (SCLC) cells, the bispecific T-cell engager tarlatamab has previously demonstrated improved survival outcomes in patients with ES-SCLC when administered as second-line monotherapy (N Engl J Med. 2025;393:349–361) or as first-line maintenance therapy with a PD-L1 inhibitor (Lancet Oncol. 2025:S1470-2045(25)00480-2).
In the DeLLphi-303 study, the tarlatamab combination demonstrated a manageable toxicity profile. Cases of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were mostly low grade and only 6% of patients discontinued treatment due to a tarlatamab-related adverse event. “Although CRS and ICANS are potential concerns, the low incidence of severe events indicates that, in experienced hands and with well-selected patients, this approach is feasible,” notes Prof. Suresh Senan from Amsterdam University Medical Centers, Netherlands.
In SCLC, the lack of predictive biomarkers means many patients, regardless of appropriateness, receive treatment to achieve limited gains. Tarlatamab also represents a step toward biomarker-guided therapy, explains Senan. “The extensive cell expression of the tarlatamab target is about as close as we currently have to a biomarker in ES-SCLC. Future translational studies will be critical to identify patients who may benefit most from its early use.”
At the ESMO Congress, promising findings were also reported for ceralasertib, a selective ataxia telangiectasia and Rad3-related kinase inhibitor that has previously shown durable responses with manageable haematologic toxicity when combined with durvalumab in a phase II study of patients with relapsed and refractory ES-SCLC (J Clin Oncol. 2024;42(Suppl. 16):8104).
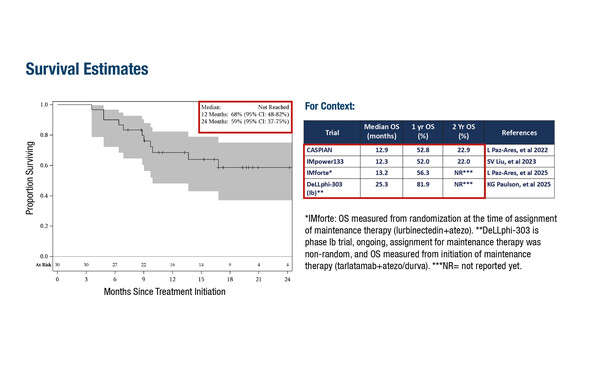
In a phase II study, ceralasertib demonstrated an ORR of 73.3%, mPFS of 6.1 months and respective 12- and 24-month OS of 68% and 59% when given as maintenance therapy in combination with durvalumab in 30 patients who had not had disease progression following platinum-based chemoimmunotherapy (Abstract 2761O, key results in the box below). The regimen was mostly associated with low-grade gastrointestinal and haematological toxicities. “Although the study population was small, the combination of ceralasertib and durvalumab appeared well tolerated with a safety profile comparable to chemo-immunotherapy,” says Senan. “The survival outcomes at 12 and 24 months are encouraging, suggesting potential benefit in this difficult-to-treat patient population and warranting further investigation.”
Looking at the future management of SCLC, Senan believes that a multi-arm phase II study comparing these novel treatment strategies may help to optimise therapy. “Additionally, results from translational research could help clarify molecular subtypes and guide personalised treatment approaches – a crucial next step in this challenging disease.” Studies to follow include the SWOG S2409 PRISM trial, which will use biomarker analysis to select and test new, personalised treatments for ES-SCLC (NCT06769126).
At a glance:
Wermke M, et al. Tarlatamab with first-line chemoimmunotherapy for extensive stage small cell lung cancer (ES-SCLC): DeLLphi-303 study. ESMO Congress 2025, Abstract 2757O
- Grade ≥3 TRAEs: 78%; 1 fatal TRAE
- ORR: 71%
- mOS: not reached
- mDOR: 11.0 mo
- mPFS: 10.3 mo
- 12-mo OS: 81%
Furqan M, et al. Chemo-immunotherapy followed by durvalumab and ceralasertib in treatment naïve patients with extensive-stage small cell lung cancer. ESMO Congress 2025, Abstract 2761O
- ORR: 73.3% (2 CR)
- mDOR: 6.6 mo
- mPFS: 6.1 mo
- 12-mo OS: 68%; 24-mo OS: 59%
- SAEs: 46.7% (23.3% were treatment-related)