In early phase studies, IAG933 and VT3989 led to encouraging disease control rates with manageable toxicity
“Mesothelioma is a highly symptomatic disease and therefore it is not only response rates that matter to patients. Disease control is clinically meaningful and the rate of stable disease observed in these two relatively large studies with TEAD inhibitors in heavily pre-treated patients was impressive,” says Prof. Alessandra Curioni-Fontecedro of the University of Fribourg and Cantonal Hospital of Fribourg, Switzerland, commenting on findings presented at the ESMO Congress 2025 (Berlin, 17–21 October).
Currently, there are very few third-line options for the treatment of mesothelioma and no approved targeted treatments. The Hippo signalling pathway, which controls tissue growth, emerged as a promising target as it is frequently inactivated in various solid tumour types, including pleural mesothelioma. The inactivation of the Hippo pathway causes the translocation of YAP/TAZ to the nucleus where they bind to TEAD transcription factors inducing gene expression (J Cell Mol Med. 2024;28:e18330).
In a first-in-human study, the oral inhibitor of the interaction between YAP/TAZ and TEADs, IAG933, seemed well tolerated and demonstrated evidence of clinical activity when given as monotherapy (Abstract 919O, key results in the box below). In 136 heavily pre-treated patients, of whom more than 80% had mesothelioma, dose-limiting toxicities (DLTs) of QTc prolongation were observed at the highest intermittent (600 mg; n=2) and continuous once-daily (400 mg; n=2) schedules. There was one DLT of grade 2 proteinuria (300 mg continuous once-daily); otherwise, albuminuria and proteinuria were generally reversible. A confirmed partial response (PR) was observed in 5 out of 30 patients with pleural mesothelioma on IAG933 300/400 mg continuous once-daily dosing (objective response rate [ORR] of 16.6%), with many achieving stable disease on these and other dosing schedules. There were confirmed PRs in 1 patient with peritoneal mesothelioma and 1 patient with epithelioid haemangioendothelioma (EHE), and an unconfirmed PR in another patient with EHE. Limited patient numbers on active dosing schedules meant that conclusions could not be drawn on the association between response and NF2 mutations, which are frequently involved in Hippo dysregulation.
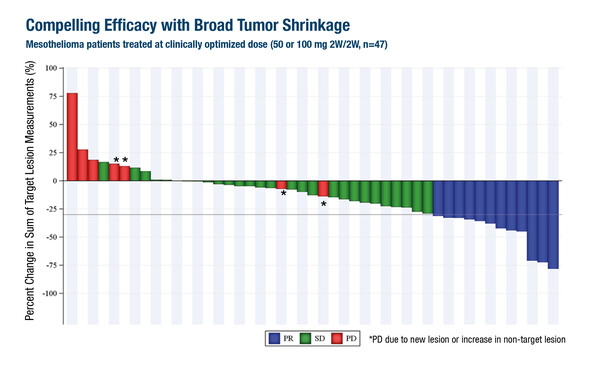
In a second phase I/II trial, the oral auto-palmitoylation inhibitor of TEAD, VT3989, demonstrated manageable safety and promising efficacy in 172 patients, almost 80% of whom had mesothelioma (Abstract 920O, key results in the box below). The median number of prior lines of therapy was 3 and 31.4% of patients had an NF2 mutation. Grade 3–4 treatment-related adverse events (AEs) occurred in 8.7% of patients across the entire population, most commonly increased urine albumin-to-creatinine ratio (1.7%), while all incidences of proteinuria was lower-grade and reversible. Among 47 patients with clinically optimised dosing (50 or 100 mg once-daily 2 weeks on/2 weeks off), 26% had a PR and 60% had stable disease. PRs were noted in patients without NF2 mutations.
“It will be intriguing to learn more about the relationship between response and NF2 mutation status to determine whether patients will need to be selected to gain benefit from TEAD inhibitors and to guide the further development of targeted treatments,” adds Curioni-Fontecedro.
While novel targeted agents showed promise, an encouraging ORR observed with durvalumab plus chemotherapy failed to translate into overall survival (OS) benefits in an analysis of the phase III DREAM3R trial also presented at the Congress (LBA104, key results in the box below). The trial compared durvalumab plus chemotherapy versus chemotherapy as first-line systemic therapy in 174 patients with unresectable pleural mesothelioma. The primary endpoint of median OS was 21 months with durvalumab plus chemotherapy and 18 months with chemotherapy alone (hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.63–1.36; p=0.90) after a median follow-up of 33 months, while median PFS was 8 months and 7 months, respectively (HR 0.70; 95% CI 0.50–0.98; p=0.20). The ORR was 58% with durvalumab plus chemotherapy compared with 35% for chemotherapy alone (p=0.005). Immune checkpoint inhibitors were used after progression by 21% of patients in the durvalumab plus chemotherapy group and 48% of patients in the chemotherapy alone group (p<0.0001). Curioni-Fontecedro puts these results into context with findings from phase III first-line trials with nivolumab plus ipilimumab (Lancet. 2021;397:375–386) and pembrolizumab added to chemotherapy (Lancet. 2023;402:2295–2306) where OS improvements were achieved: “The findings from DREAM3R may be due to the fact that only 7% of patients had non-epithelioid pleural mesothelioma, while in the other trials, the overall benefit may have been driven by at least 20% of patients with the non-epithelioid subtype. In addition, OS was high in both arms in DREAM3R, likely representing survival improvements over recent years and the sequential benefit that patients in the chemotherapy alone arm gained from receiving subsequent immunotherapy.”
Whether the future of mesothelioma treatment lies with targeted therapy or immunomodulators, such as bispecific antibodies, is uncertain, concludes Curioni-Fontecedro: “In each case, further large trials and biomarker analyses will be needed to identify the right populations for the right treatments.”
At a glance:
Garralda E, et al. A first-in-human study of oral IAG933 in adult patients with advanced mesothelioma and other solid tumours. ESMO Congress 2025 - Abstract 919O
- N=136, 83.8% with mesothelioma
- MTD: 300 mg continuous qd schedule
- Grade ≥3 TRAEs: 5.7% with continuous qd dosing
- ORR: 16.6% among 30 pts with pleural mesothelioma on continuous qd dosing
Yap TA, et al. Safety and efficacy of first-in-class, YAP/TEAD inhibitor, VT3989 in refractory pleural and non-pleural mesothelioma: A phase I/II study. ESMO Congress 2025 - Abstract 920O
- N=172, 78.5% with mesothelioma
- Grade ≥3 TRAEs: 8.7%
- mPFS: 25 wks in 47 pts with clinically optimised dose regardless of UACR
- mPFS: 40 wks in 22 pts with clinically optimised dose and UACR dose modification
Nowak AK, et al. Primary results of DREAM3R: DuRvalumab (MEDI4736) with chEmotherapy as first line treAtment in advanced pleural Mesothelioma - A phase 3 Randomised trial. ESMO Congress 2025 - LBA104
- N=174 (durvalumab + CT n=114; CT n=60)
- Durvalumab + CT vs CT:
- mOS: 21 mo vs 18 mo: HR 0.92; 95% CI 0.63–1.36; p=0.90
- mPFS: 8 mo vs 7 mo: HR 0.70; 95% CI 0.50–0.98; p=0.20
- ORR: 58% vs 35%; p=0.005
- Grade 3–5 AEs: 51% vs 40%