New positive findings from research in advanced or metastatic breast cancer support the use of these agents in wider patient populations
Antibody–drug conjugates (ADCs) have expanded the treatment landscape for patients with unresectable locally advanced or metastatic breast cancer, providing new therapeutic options where other treatments have failed. Some of the latest research presented at the ESMO Congress 2024 (Barcelona, 13–17 September) was on broadening the use of current ADCs to a wider patient population, as well as exploring novel ADCs in advanced or metastatic breast cancer, providing new insights into targeting HER2 and HER3 to achieve better outcomes for patients.
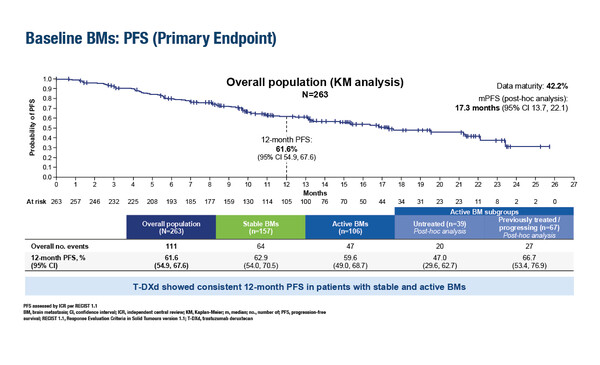
Late-Breaking primary results from the phase IIIb/IV, open-label DESTINYBreast-12 trial revealed substantial and durable efficacy in 504 patients with HER2-positive metastatic breast cancer, including those with brain metastases (LBA18). In the cohort with brain metastases (n=263), the 12-month progression-free survival (PFS) rate was 61.6%, while the 12-month central nervous system (CNS) PFS was 58.9%, with similar results in those with stable (PFS, 57.8%) and active (PFS, 60.1%) brain metastases. The 504 patients enrolled in the open-label trial had experienced disease progression on ≤2 prior lines of therapy.
Commenting on these results, Dr Philippe Aftimos from Institut Jules Bordet – Université Libre de Bruxelles, Brussels, Belgium, says, “We have anticipated these results with great interest as the incidence of brain metastases in HER2-positive metastatic breast cancer is common and until recently, most patients would have required radiation therapy that could be associated with significant toxicity.” He explains that initial reservations over the use of trastuzumab deruxtecan in patients with untreated brain metastases appear to be unfounded, thanks to these results. “While trastuzumab deruxtecan is indicated for HER2-positive advanced/metastatic breast cancer, there were fears that a molecule the size of trastuzumab deruxtecan would be too large to cross the blood–brain barrier and so would not treat brain metastases. However, the results of DESTINYBreast-12 have shown that the outcome for patients with brain metastases was positive.” Aftimos adds that these results suggest that a systemic strategy could be effective in treating advanced breast cancer with brain metastases without the additional need for local therapy.
At the Congress, investigators from France shared the results of the phase II, single-arm ICARUS-BREAST01 study, which investigated the novel anti-HER3 monoclonal antibody-containing ADC, patritumab deruxtecan, in patients with hormone receptor (HR)-positive/HER2-negative unresectable locally advanced/metastatic breast cancer who had progressed on CDK-4/6 inhibitors plus endocrine therapy and one line of chemotherapy (Abstract 340O). At the data cut-off, 99 patients had received treatment and at a median follow-up of 15.3 months, the confirmed objective response rate was 53.5% and median PFS was 9.4 months. “While the importance of HER3 as a target is yet to be fully explored, it is known that HER3 expression is associated with poor prognosis,” says Aftimos, adding that the efficacy of patritumab deruxtecan appears to be compelling. Around half of patients (50.1%) had treatment-related grade ≥3 adverse events; the most frequently reported events, regardless of grade, were fatigue, nausea and diarrhoea. Aftimos comments, “We have clinical experience of managing adverse events like these pre-emptively, therefore based on the early data available, the tolerability of patritumab deruxtecan would be manageable in a clinical setting.” With parallel consideration of the recent results for trastuzumab deruxtecan in patients with hormone receptor (HR)-positive/HER2-low or -ultralow metastatic breast cancer in the DESTINY-Breast06 trial, where 90.4% of patients had prior CKD-4/6 therapy (J Clin Oncol. 2024;42(Suppl):LBA1000), Aftimos says, “These encouraging early results for patritumab deruxtecan suggest it may be a future option after failure of CDK-4/6 inhibitor therapy in HR-positive/HER2-negative advanced breast cancer and we eagerly await further clinical data.”
Aftimos explains that further data are required regarding the future integration of ADCs in clinical practice, and that several challenges remain. “While ADCs are already approved for metastatic breast cancer, and if ongoing clinical trials investigating their use in the early disease setting continue to provide positive results, we would need to find an optimal way to introduce novel ADCs into the paradigm of treatment with curative intent, while balancing and managing their side effects,” he says. “Also, there is the potential for multiple ADC options in the breast cancer setting, which raises the issue of treatment sequencing.” Currently, two novel ADCs have been approved for advanced/metastatic breast cancer – trastuzumab deruxtecan for HER2-positive and HER2-low disease (trastuzumab deruxtecan) and sacituzumab govitecan for heavily pre-treated triple-negative disease and HR-positive/HER2-negative disease (sacituzumab govitecan).
According to Aftimos, there is also a need for further understanding of biomarkers and their link to outcomes, as well as the opportunities for a broader variety of patients to be eligible for ADC treatment, as observed in the positive results for HER2-ultralow in the DESTINY-Breast06 study, and a better understanding of HER3 as a target. “From my perspective, it is exciting to see new evidence for ADCs being presented for advanced and metastatic breast cancer, and there is still much more research to come as we explore their role in earlier treatment lines. The ongoing research into ADCs holds great promise for future opportunities to offer personalised, effective options to improve outcomes for more patients,” he concludes.
Programme details
Lin N, et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2+ advanced/metastatic breast cancer (mBC) with or without brain metastases (BM): DESTINYBreast-12 primary results. ESMO Congress 2024, LBA18
Proffered Paper Session – Breast cancer, metastatic, 13.09.2024, h. 16:00 – 17:30, Barcelona Auditorium – Hall 2
Pistilli B, et al. Efficacy, safety and biomarker analysis of ICARUS-BREAST01: A phase 2 study of patritumab deruxtecan (HER3-DXd) in patients (pts) with HR+/HER2- advanced breast cancer (ABC). ESMO Congress 2024, Abstract 340O
Proffered Paper Session - Breast cancer, metastatic, 13.09.2024, h. 16:00 – 17:30, Barcelona Auditorium – Hall 2