Alectinib is the first ALK inhibitor to significantly improve DFS in a phase III trial across disease stages
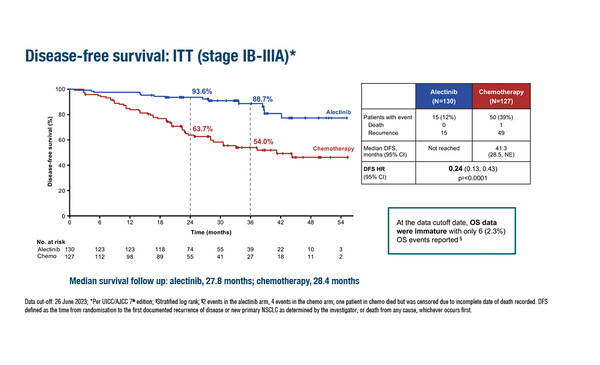
Practice-changing findings from a next-generation ALK inhibitor were presented for patients with completely resected ALK-positive non-small cell lung cancer (NSCLC) at a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October). According to an interim analysis of the ALINA trial, adjuvant targeted treatment with alectinib was associated with significant disease-free survival (DFS) benefits compared with platinum-based chemotherapy, with favourable results for alectinib seen in both the stage II–IIIA population (n=231; hazard ratio [HR] 0.24; 95% confidence interval [CI] 0.13–0.45; p<0.0001) and the intention-to-treat (ITT) (stage IB–IIIA) population (n=257; HR 0.24; 95% CI 0.13–0.43; p<0.0001) (LBA2). The median follow-up was 27.8–27.9 months across both populations. Two-year DFS rates with alectinib and chemotherapy were 93.8% versus 63.0%, respectively, in the stage II–IIIA population and 93.6% versus 63.7%, respectively, in the ITT population.
Grade 3–4 adverse events (AEs) were reported in 30% of patients receiving alectinib and 31% receiving chemotherapy. There were no grade 5 AEs in either treatment arm. Overall, 13% of patients receiving alectinib had serious AEs compared with 8% receiving chemotherapy, but the rates of serious treatment-related AEs and AEs leading to treatment withdrawal were lower with alectinib (2% and 5%, respectively) compared with chemotherapy (7% and 13%, respectively).
As reflected in the ESMO Clinical Practice Guideline for the diagnosis, treatment and follow-up of oncogene-addicted metastatic NSCLC, targeted therapies are known to be superior to conventional chemotherapy in ALK-positive metastatic NSCLC (Ann Oncol. 2023;34:339–357), but so far, there have been very few data from patients with resected ALK-positive disease. “The findings from the ALINA trial will absolutely help to change practice as they give a very clear signal, in a head-to-head comparison with chemotherapy, that adjuvant targeted treatment with alectinib improves DFS in this setting. And the analysis shows that alectinib is effective across all stages of disease,” says Prof. Martin Reck from the Lung Clinic Grosshansdorf, Germany.
Alectinib also led to a clinically meaningful CNS-DFS benefit compared with chemotherapy in the ITT population (HR 0.22; 95% CI 0.08–0.58). “Central nervous system data are incredibly important,” continues Reck. “The brain is a common area of relapse following resection and patients with ALK-positive NSCLC appear to be at particular risk of developing brain metastases (Oncotarget. 2018;9:35181–35194). We need a drug that works not only on the lung but also on the brain, and this is what the trial demonstrates for alectinib.”
Despite the positive results presented, overall survival data are still immature and long-term data are required to assess the impact of alectinib on prognosis. “In addition, we need to look at biomarkers and to investigate if the effect of alectinib is homogeneous across ALK translocations or if there is a different variant partner, or a specific variant, that is indicative of a particularly good response – or, conversely, a detrimental effect – with treatment,” concludes Reck. “Finally, it will be important to define how we treat patients with resected ALK-positive NSCLC who relapse following adjuvant alectinib.”