Promising data from the ATTLAS phase III study indicate a potential new approach for treating patients progressing on TKIs
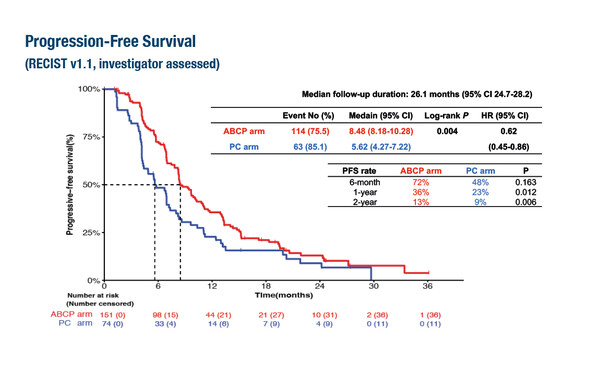
As presented at the ESMO Congress 2023 (Madrid, 20–24 October), ATTLAS, the first randomised phase III trial of atezolizumab, bevacizumab and chemotherapy (ABCP) in patients with non-small cell lung cancer (NSCLC) and acquired resistance to TKIs, met its primary endpoint, with significantly longer median progression-free survival (PFS) in patients treated with ABCP compared with chemotherapy alone (8.5 months versus 5.6 months, hazard ratio [HR] 0.62; 95% confidence interval 0.45–0.86; p=0.004) (LBA67).
“The ATTLAS study represents part of the ongoing research effort focused on the unmet need for novel treatments in patients who have progressed on TKIs, and these results confirm findings from previous preliminary data in subgroup analyses of the IMpower150 study,” says Dr Daniel Tan from the National Cancer Centre Singapore. In the IMpower 150 study, a potential survival benefit was reported for patients with EGFR mutations and previous TKI therapy treated with the combination of a PD-L1 inhibitor and a VEGF inhibitor (Lancet Respir Med. 2019;75:3887–3401).
In the ATTLAS trial, consistent with the PFS benefit, objective response rates were higher for patients treated with ABCP compared with chemotherapy (69.5% versus 41.9%, respectively; p<0.001). No additional safety signals were reported. The trial enrolled 228 patients with activating EGFR mutations or ALK translocations who had experienced disease progression on prior TKI therapy.
The impact on PFS was correlated with PD-L1 expression, with HRs of 0.47, 0.41 and 0.24 reported for patients with PD-L1 ≥1%, ≥10% and ≥50%, respectively. Commenting on these findings, Tan remarks, “This observation is interesting as in previous studies we have seen that PD-L1 expression does not have a strong association with response to checkpoint inhibitors in patients with driver mutations. However, further validation in future trials will be required to achieve a greater understanding of this.”
While TKIs are the established standard of care for NSCLC harbouring driver mutations, most patients ultimately develop resistance to these treatments. The ATTLAS study is the first prospective trial suggesting some benefit to using this quadruple drug regimen compared with chemotherapy.
Looking ahead, Tan notes, “There are many interesting studies ongoing in patients with driver mutations and acquired TKI resistance, including studies of fourth-generation EGFR inhibitors, antibody–drug conjugates directed at HER3 and TROP2 and combinatorial strategies with c-MET inhibitors. Given that response rates typically range from 30–50% for NSCLC treatments, one of the key challenges will be to develop biomarkers that allow the precise targeting of treatments to the patients who are most likely to respond. We also need to consider the timing of the transition between TKIs and immunotherapy combinations, due to potential for adverse events. As such, there needs to be careful consideration about the appropriate timing of TKI discontinuation to optimise treatment benefit for patients.”
Abstract discussed:
Ahn M-J, et al. A phase 3, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR or ALK mutated in non-small cell lung cancer (ATTLAS, KCSG-LU19-04). ESMO Congress 2023, LBA67
Proffered Paper Session – NSCLC, metastatic, 20.10.2023, h. 16:00 – 17:30, Barcelona Auditorium – Hall 9