Results from the LIBRETTO-431 trial reinforce the use of selpercatinib as the first-line choice and are likely to widen patient access to treatment
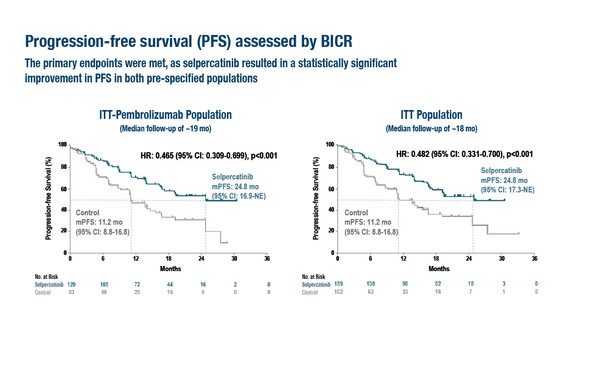
As presented in a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October), long-awaited results from the randomised phase III LIBRETTO-431 trial in RET fusion-positive advanced/metastatic non-small cell lung cancer (NSCLC) reported significant prolongation of the primary endpoint, progression-free survival (PFS), with the selective RET inhibitor selpercatinib compared with platinum and pemetrexed chemotherapy plus pembrolizumab (intention-to-treat [ITT]-pembro population) and chemotherapy plus or minus pembrolizumab (n=261; ITT population) (LBA4). The median PFS in the ITT-pembro population was 24.8 months with selpercatinib versus 11.2 months with control (hazard ratio [HR] 0.465; 95% confidence interval [CI] 0.309–0.699; p<0.001) at a median follow-up of approximately 19 months. PFS benefits of selpercatinib compared with control in the ITT population closely mirrored those seen in the ITT-pembro population (HR 0.482; 95% CI 0.331–0.700; p<0.001). Clinically meaningful improvements in objective response rate, duration of response and intracranial response were noted with selpercatinib versus control. Selpercatinib also prolonged the time to CNS progression versus control (cause-specific HR 0.28; 95% CI 0.12–0.68). Adverse events observed with selpercatinib and in the control arm were generally consistent with those previously reported.
In the LIBRETTO-431 trial, PFS was tested first in the ITT-pembro population and then in the ITT population. Crossover to selpercatinib was allowed following blinded independent central review-assessed progression in the control arm.
“These highly anticipated results are tremendously exciting and further establish the use of RET inhibition upfront as the primary approach for patients with RET fusion-positive NSCLC,” says Prof. Sanjay Popat from the Royal Marsden Hospital and the Institute of Cancer Research, London, UK, commenting on the data presented reinforcing the efficacy reported in the phase I/II LIBRETTO-001 trial (J Clin Oncol. 2023;41:385–394). “Selpercatinib almost doubled the PFS compared with chemotherapy, with or without immunotherapy. Indeed, the HRs in the ITT-pembro and ITT cohorts are very similar, suggesting that the addition of pembrolizumab to the control arm does not improve its efficacy, although more scrutiny of the results will be required to confirm this.” According to Popat, a very positive finding for patients is the 72% improvement in the time to CNS relapse because brain metastases can have a considerable impact on both their well-being and their quality of life.
In terms of the choice of RET inhibitors, Popat says: “The results presented at the ESMO Congress 2023 indicate that the weight of data is currently behind selpercatinib. However, results from a confirmatory phase III trial of the similar selective RET inhibitor, pralsetinib, are awaited in late 2024 and may influence the choice of RET inhibitors in clinical practice (NCT04222972). In the meantime, the good news for patients is that selpercatinib is now more likely to be reimbursed based on the LIBRETTO-431 trial results in some areas of Europe where this had been withheld pending confirmation of efficacy in a phase III trial. It will mean that more patients should be able to benefit from this treatment approach.”
Finally, Popat observes that the study data reinforce the need for RET testing at the time of diagnosis of NSCLC. “Given that most patients will eventually relapse on RET inhibitors, we also look forward to additional datasets from the trial to help us understand the magnitude of on- and off-target acquired resistance to selpercatinib in the search for subsequent treatment strategies,” he concludes.