Phase II data report a pathologic complete response in patients who received radiotherapy after neoadjuvant therapy without surgery, when selected by image-guided vacuum-assisted core biopsy
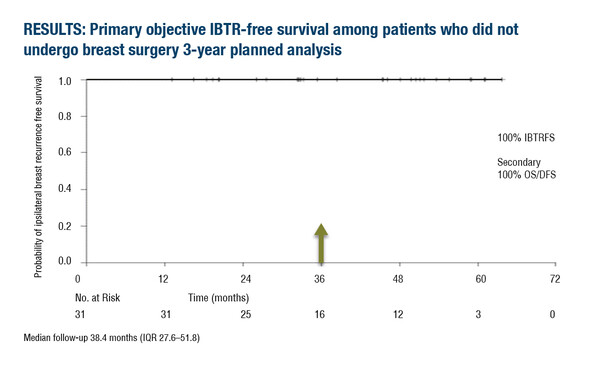
Highly selected patients with breast cancer who underwent radiotherapy, but not surgery, reported a 0% ipsilateral breast tumour recurrence according to the results of a pre-planned, 3-year primary endpoint of a multicentre, prospective phase II trial presented at the ESMO Congress 2023 (Madrid, 20–24 October) (Abstract 243MO).
Following the promising outcomes of a feasibility study (J Am Coll Surg. 2023;237:101–108; Lancet Oncol. 2022;23:1517–1524), researchers investigated the benefits of omitting surgery after neoadjuvant systemic therapy (NST). The trial involved women with unicentric cT1-2N0-1M0 triple negative breast cancer (TNBC) or HER2-positive breast cancer and a residual breast lesion <2 cm on imaging after NST. In patients without invasive/in situ disease on a minimum 12-core 9G image-guided vacuum-assisted core biopsy (VACB) from the tumour bed, breast surgery was omitted and patients instead underwent whole-breast radiotherapy/boost.
Among 31 patients with VACB-determined pathologic complete response after NST, no recurrence was reported at a median follow-up of 38.4 months (interquartile range 27.6–51.8) and 3-year rates of disease-free and overall survival were 100%. Two patients were positive for circulating tumour cells (CTCs) at baseline, two were positive at 6 months, and one was positive at 12 months. No patients had CTCs detected at more than one timepoint.
The 50 patients enrolled in the study had a mean age of 60.4 years; 21 had TNBC and 29 had HER2-positive disease. The mean post-NST imaging tumour size was 0.90 cm (standard deviation 0.81) and 17 patients (34%) had a complete radiologic response.
The positive findings provide supporting evidence that image-guided VACB can be used to effectively select distinct patients who can safely receive radiotherapy without surgery. However, additional follow-up and further confirmation from clinical trials are needed before this approach becomes standard in clinical practice.
Abstract discussed:
Kuerer HM, et al. Omission of breast surgery after neoadjuvant systemic therapy for invasive cancer: three-year preplanned primary-endpoint on a phase 2 multicentre prospective trial. ESMO Congress 2023, Abstract 243MO
Mini Oral Session – Breast cancer, early stage, 23.10.2023, h. 10.15 – 11.45, Bilbao Auditorium, NCC