IMmotion010, CheckMate 914 and PROSPER miss their primary endpoints: back to square one for adjuvant immunotherapy?
At ESMO Congress 2022, results from three phase III trials evaluating the use of adjuvant immunotherapy in renal cell carcinoma (RCC) show no improvements in disease-free survival (DFS) in patients enrolled, opening up new questions on the future of immunotherapy in this setting.
In the first study, the IMmotion010 phase III trial, the anti-PD-L1 antibody atezolizumab (1,200 mg IV) was evaluated versus placebo as adjuvant treatment in 778 patients with RCC who had an increased risk of recurrence (LBA66). After a median follow-up of 44.7 months, median investigator-assessed DFS was 57.2 months (95% confidence interval [CI] 44.6–not estimable [NE]) for atezolizumab and 49.5 months (95% CI 47.4–NE) for placebo (hazard ratio [HR] 0.93; 95% CI 0.75–1.15; p=0.495). No between-arm differences were observed in DFS in patients with PD-L1≥1% (HR 0.83; 95% CI 0.63–1.10) or in overall survival in patients with PD-L1 1% to <5% (HR 0.92; 95% CI 0.68–1.25). In terms of safety, 27.2% and 21.1% of patients had grade 3–4 adverse events with atezolizumab and placebo, respectively.
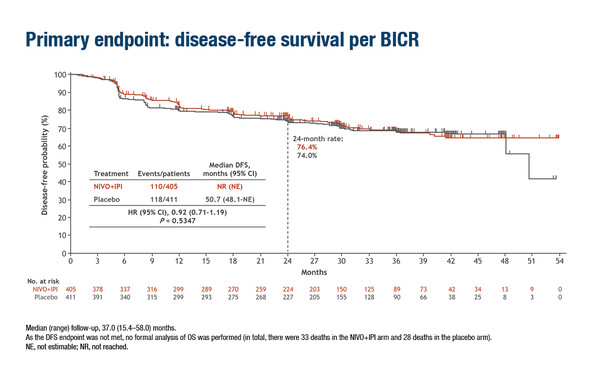
Similarly, no significant difference was noted in DFS in part A of the CheckMate 914 trial that evaluated adjuvant nivolumab – an anti-PD-1 antibody – plus ipilimumab – an anti-CTLA-4 antibody – versus placebo in patients with RCC and a high risk of relapse (LBA4). Median DFS by blinded central review was not reached with nivolumab plus ipilimumab versus 50.7 months with placebo (HR 0.92; 95% CI 0.71–1.19; p=0.5347). DFS probability at 24 months was 76.4% and 74.0%, respectively. A greater proportion of patients receiving nivolumab plus ipilimumab experienced grade ≥3 adverse events (28%) compared with placebo (2%).
The PROSPER trial was slightly different, with one dose of nivolumab given prior to surgery and nine doses given after nephrectomy (LBA67). The study randomised 819 patients and the primary endpoint was recurrence-free survival (RFS). The trial was stopped early by the data and safety monitoring committee due to futility. RFS was similar in both arms (HR 0.97; 95% CI 0.74–1.28; p=0.43). One-fifth of patients treated with nivolumab experienced at least one grade 3–4 adverse event that could be attributable to nivolumab, compared with 6% in the control arm. The incidence of surgical complications was similar in both arms.
“Expectations for positive results from these three trials were high, given the previous positive DFS data in this disease setting with pembrolizumab in the KEYNOTE-564 trial,” says Dr Dominik Berthold of the Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, commenting on the data, “The negative results are unexpected.” In 2021, pembrolizumab was approved as adjuvant therapy in RCC after the encouraging results of the prespecified interim analysis of the KEYNOTE-564 trial with DFS at 24 months that was significantly greater with pembrolizumab (77.3%) compared with placebo (68.1%) (HR 0.68; 95% CI 0.53–0.87; p=0.002) (N Engl J Med. 2021;385:683–694). Berthold continues, “At the moment, reasons for failure of the IMmotion010, CheckMate 914 and PROSPER trials are unclear, but could reflect differences in the patient populations, such as the proportions of patients with very advanced or resected metastatic disease, the different mechanism of action of the respective immunotherapy agents tested or differences in follow-up times. Possibly, the nature of randomised trials means there may be a statistical outlier, albeit the likelihood of this is small. Subgroup analyses using data from all three phase III trials are important to tease out which patients will have the most benefit – and to investigate the presence or absence of predictive biomarkers.”
How will these outcomes impact patients with RCC? “It is encouraging that the safety findings with immunotherapy shown in these studies are within expected ranges,” says Berthold. “An important consideration is how the latest data will impact the use of pembrolizumab. For instance, patients may be less likely to consider adjuvant treatment – and the associated side-effects – if the number of negative trials outweighs the positive trials.”
Berthold concludes, “We are still missing some important information, such as the impact of follow-up on overall survival, which was a secondary endpoint in all trials. This is something to figure out over time. Another important question relates to the use of immunotherapy as neoadjuvant treatment, potentially combined with other agents. There are more data to come and much more still to learn about how to prevent recurrence in these patients.”
Abstracts presented:
Bex A, et al. IMmotion010: efficacy and safety from the phase III study of atezolizumab (atezo) vs placebo (pbo) as adjuvant therapy in patients with renal cell carcinoma (RCC) at increased risk of recurrence after resection. ESMO Congress 2022, LBA66
Proffered Paper Session 1 – GU tumours, non-prostate, 10.09.2022, h. 10:15 – 11:45, Brest Auditorium
Motzer RJ, et al. Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: results from the randomized, phase III CheckMate 914 trial. ESMO Congress 2022, LBA4
Presidential Symposium II, 11.09.2022, h. 16:30 – 18:15, Paris Auditorium
Allaf M, et al. Phase III RandOmized Study comparing PErioperative nivolumab (nivo) versus observation in patients (Pts) with Renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. ESMO Congress 2022, LBA67
Proffered Paper Session 1 – GU tumours, non-prostate, 10.09.2022, h. 10:15 – 11:45, Brest Auditorium