In a study presented at ESMO Congress 2022, air pollution is shown to drive interleukin-1β release in cells with EGFR mutations to promote lung cancer development
Compelling evidence exists to link air pollution exposure with lung cancer incidence and mortality (Front Med 2021;8:742076; Thorax 2016;71:891–898), with more than 300,000 lung cancer deaths globally linked to exposure to ambient (outdoor) air pollution in 2019 (Front Med 2021;8:742076). Until now, however, the molecular mechanism underlying the link between air pollution and lung cancer in non-smokers had not been elucidated.
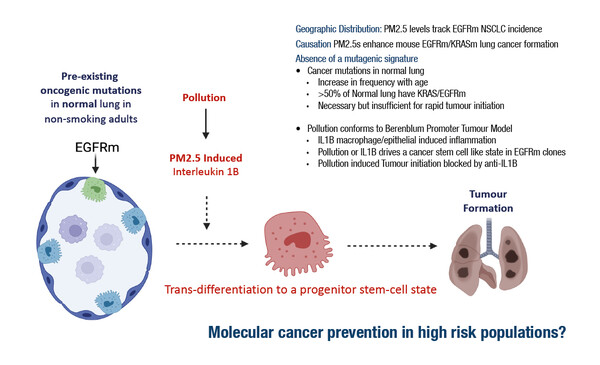
According to late-breaking data presented at ESMO Congress 2022 (LBA 1), increasing exposure to 2.5 µm particulate matter (PM2.5) increases the risk of non-small-cell lung cancer in non-smoking individuals with EGFR mutations. Researchers from the Francis Crick Institute leading the study explained that this effect is driven by an influx of macrophages and an increase in the inflammatory mediator interleukin-1β, which promotes carcinogenesis in airway cells. Approximately half of non-smokers with lung cancer have EGFR mutations in their cancer cells.
The study involved 447,932 individuals to address the associations of increasing 2.5um PM (PM2.5) concentrations with cancer risk. In a final series of experiments, the Crick Institute researchers used state-of-the-art, ultradeep mutational profiling of 247 small samples of normal lung tissue and found EGFR and KRAS driver mutations in 15% and 53% of normal lung samples, respectively. According to the research team, driver mutations in EGFR and KRAS genes are a likely consequence of ageing and only weakly potentiate cancer in laboratory models.
However, when lung cells with these mutations were exposed to air pollutants, carcinogenesis occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes a macrophage response and a progenitor-like state in lung epithelium harbouring mutant EGFR. The laboratory findings now raise the question of whether these data could pave the way to new molecular-based prevention approaches and development of targeted therapies for non-smokers who are at-risk of lung cancer i.e., those harbouring EGFR mutations.
Commenting on the potential implications of these findings, Prof. Tony Mok of the Chinese University of Hong Kong says that the promise of molecular screening for non-smokers is still premature. “We can say that in the future, it may be possible to further improve cancer prevention by first identifying non-smokers with EGFR mutations in their cells before using low-dose computed tomography (LD-CT) to screen for presence of lesions or pre-malignant ground-glass opacity. Before this, we need to determine if the presence of EGFR mutations can be detected in normal cells in non-smokers using ultrasensitive methods and whether LD-CT can detect premalignant changes. Future research could investigate whether these lesions can be targeted with interleukin-1β inhibitors. There are still a lot of unknowns, but these data are exciting and intriguing and may signal a new era of chemoprevention in lung cancer.”
Currently, annual screening for lung cancer using LD-CT is generally recommended in individuals aged 55–80 years with a history of ≥30 pack-years of smoking who are current smokers or stopped smoking within the past 15 years. Using an LD-CT protocol, lung cancer mortality in smokers was significantly reduced by 20% in the National Lung Screening Trial, compared with use of chest radiography (N Engl J Med 2011;365:395–409). For non-smokers, the Taiwan Lung Cancer Screening for Never Smoker Trial (TALENT) used LD-CT to screen high-risk individuals – including those with a family history of lung cancer, second-hand smoking exposure, or presence of lung disease – leading to a diagnosis of lung cancer in 2.6% of participants (J Thorac Oncol. 2021;16(Suppl):S58).
“The data from TALENT suggest that early-stage lung cancer can be detected in non-smokers using LD-CT screening. We know there are barriers to widespread LD-CT screening relating to cost and logistics such as availability of CT scanners, number of clinicians and radiographers required to interpret the findings, and definitions of ‘at-risk’ populations,” says Mok.
Returning to the importance of controlling air pollution in order to improve health outcomes, Mok notes, “Any screening programme would go hand-in-hand with the control of carbon emissions and consumption of fossil fuels, and we are hopefully now more conscious than ever of the importance of these factors. Controlling environmental pollution is largely a matter for policymakers, which will hopefully result in reduction of PM2.5 levels. Lung cancer prevention is one of many reasons to control air pollution.” While knowledge of this setting is evolving, it is important that scientific observations progress to biological explanations and, ultimately, interventions. “Following on from the observation that air pollution is linked to lung cancer, these new data provide an explanation – the biological missing link – for the reason behind this, which may lead to appropriate interventions in the future. We are heading in the right direction but there is certainly a long way to go,” concludes Mok.
Abstract presented:
Swanton C, et al. Mechanism of Action and an Actionable Inflammatory Axis for Air Pollution Induced Non-Small Cell Lung Cancer: Towards Molecular Cancer Prevention. ESMO Congress 2022, LBA 1
Presidential Symposium 1, 10.09.2022, h. 16:30 – 18:00, Paris Auditorium