Patient-reported data from the DESTINY-BREAST04 and TROPiCS-02 trials may help improve current knowledge of quality of life on long-term treatment
Patient-reported outcome (PRO) data from two trials of antibody–drug conjugates (ADCs) in metastatic breast cancer (MBC) were presented at ESMO Congress 2022, improving physician’s understanding on the impact of adverse events on quality of life (QoL) of patients undergoing long-term treatments.
PROs from the randomised, phase III, DESTINY-BREAST04 trial of the ADC trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) showed that patients who received T-DXd maintained global health status (GHS)/QoL while on therapy for longer than those who received TPC in all prespecified subscales of the questionnaire (EORTC-QLQ-C30) (Abstract 217O). Median time to definitive deterioration of EORTC-QLQ-C30 GHS/QoL was 7.6 months for the T-DXd arm versus 5.1 months for the TPC arm (hazard ratio 0.71 [95% confidence interval, CI 0.56–0.92]). These data complement the primary results from this trial (N Engl J Med. 2022;387:9–20), which showed improved progression-free survival (PFS) and overall survival in patients with HER2-low MBC, regardless of hormone receptor (HR) status, with no new safety signals.
Dr Barbara Pistilli, from the Gustave Roussy Cancer Center, Villejuif, France, thinks the PRO data from this breakthrough trial are invaluable. “The investigators in this trial should be praised for evaluating different dimensions of QoL to give a comprehensive assessment, including EORTC-QLQ-C30 GHS, functioning and symptom scales, along with the breast cancer-specific QLQ-BR23, and self-reported health status using the Euro-QoL 5-dimension questionnaire, with assessments performed across the treatment period,” she explains. “PROs provide important complementary data to adverse events and their impact on patients’ QoL. Patients with MBC are living longer thanks to more effective ADC strategies and can remain on treatment for many years. It is therefore important to evaluate these patients’ perspectives on QoL over their entire treatment journey, from the point of diagnosis, and to repeatedly collect PRO data from patients receiving different treatments. Better understanding of the QoL impact of treatments will enable physicians to target specific adverse events and symptoms that are negatively affecting QoL, helping to improve treatment adherence and maintain patients on treatment.”
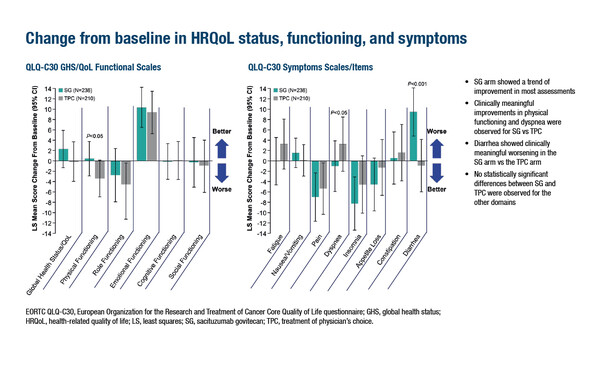
Health-related QoL (HRQoL) data from the phase III TROPiCS-02 trial of another ADC, sacituzumab govitecan (SG), versus TPC in patients with HR-positive/HER2-negative MBC were presented at a Proffered Paper Session (Abstract 1553O). A trend of improvement from baseline (including on-treatment data up to cycle 11) in most HRQoL domains in the EORTC-QLQ-C30 was observed in the SG arm, along with a statistically significant and clinically meaningful improvements in physical functioning and dyspnoea than in the TPC arm, although patients in the SG arm experienced greater worsening in diarrhoea (least-squares mean changes from baseline [95% CI] for SG versus TPC were 3.9 [0.9, 6.9], -4.3 [-8.5, -0.1] and 10.4 [6.3, 14.5], respectively; positive values indicate improvement for functioning and worsening for symptoms).
While Pistilli points out that only a 1.5-month improvement in PFS has been reported with SG versus TPC (J Clin Oncol. 2022;40:17), she acknowledges the contrast in patient populations between TROPiCS-02 and DESTINY-BREAST04. “In TROPiCS-02, the population is more heavily pre-treated than in DESTINY-BREAST04. Additionally, in TROPiCS-02, QoL is only assessed via EORTC-QLQ-C30, but importantly GHS was evaluated along with functioning and symptom scales, and the authors should be commended for striving to understand how worsening diarrhoea impacted on QoL.” Data from the planned second interim overall survival analysis and EORTC-QLQ-C30 time to deterioration endpoints from the TROPiCS-02 study were also presented at ESMO Congress 2022 (LBA76).
According to Pistilli, an additional two ADCs that are in late-phase clinical development, both of which are linked to a topoisomerase inhibitor payload, are of particular interest today. The first one is datopotamab deruxtecan, an ADC directed against trophoblast cell surface antigen-2 (Trop-2) which has been tested in different cancers. Very promising activity for this ADC have been observed in the phase I TROPION-PanTumor01 study in patients with metastatic triple-negative breast cancer (TNBC). This is now being evaluated in phase III trials versus TPC in metastatic TNBC (NCT05374512) and in HR-positive/HER2-negative MBC (NCT05104866). The second ADC to note is patritumab deruxtecan, which targets HER3. This has been tested in a phase I/II trial in HER2-positive, TNBC and HR-positive/HER2-negative MBC with promising activity in all three settings (J Clin Oncol. 2022.40;16). “It will be interesting to see QoL data from phase III trials of these ADCs, which are expected in a few years,” concludes Pistilli.
Abstracts presented:
Ueno NT, et al. Patient-reported outcomes (PROs) from DESTINY-Breast04, a randomized phase 3 study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low metastatic breast cancer (MBC). ESMO Congress 2022, Abstract 217O
Proffered Paper Session, 11.09.2022, h. 08:30-10:00, Urval Auditorium
Rugo HS, et al. Health-related quality of life (HRQoL) in the phase 3 TROPICS-02 trial of sacituzumab govitecan (SG) vs chemotherapy in HR+/HER2- metastatic breast cancer (MBC). ESMO Congress 2022, Abstract 1553O
Proffered Paper Session, 11.09.2022, h. 08:30-10:00, Urval Auditorium