Phase 1 trial data highlights preliminary efficacy and manageable safety of B7-H4–targeted antibody-drug conjugate in women receiving at least one prior topoisomerase-1 ADC
Preliminary data from the dose-escalation and backfill cohorts of an ongoing phase 1 trial, presented at the ESMO Breast Cancer 2025 (Munich, 14–17 May), demonstrated early clinical activity and manageable safety for the investigational antibody-drug conjugate (ADC) Emi-Le (emiltatug ledadotin; XMT-1660) in patients with locally advanced or metastatic triple-negative breast cancer (TNBC) who had received 1-4 prior lines of treatment, including at least one prior topoisomerase-1 ADC (Abstract 298MO).
Emi-Le is an ADC targeting B7-H4, a clinically validated target and negative prognostic factor which is highly expressed in a range of solid tumours, including breast cancers, but has limited expression in healthy tissue. It uses a proprietary auristatin F-HPA payload designed to selectively kill cancer cells while minimising off-target effects.
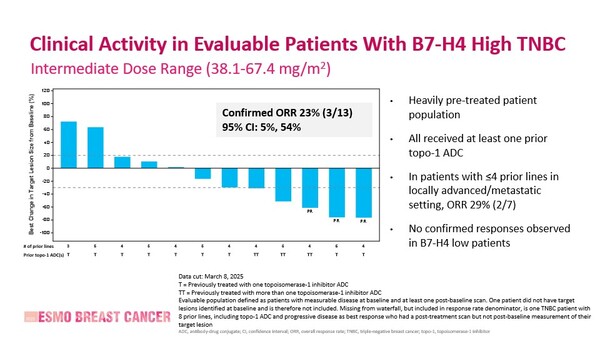
The novel ADC is being investigated as a monotherapy in a phase 1b trial, which includes both dose-escalation and dose-expansion phases across multiple tumour types. Interim results were presented from the TNBC dose escalation/backfill cohorts dosed in the intermediate dose range (38.1-67.4 mg/m2)
At data cutoff (March 8, 2025), 44 patients with TNBC had been treated in the intermediate dose range (38.1-67.4 mg/m2) and 38.9% were determined to be B7-H4 high, based on a Tumor Proportion Score (TPS) cutoff of ≥70%. The interim analysis reported an overall response rate (ORR) of 23% in evaluable patients with B7-H4 high, with no confirmed responses observed in B7-H4 low patients (Figure). More favourable outcomes in terms of preliminary progression-free survival (PFS) and overall survival (OS) were observed in patients who had received ≤4 prior lines of treatment compared to those who had received >4 prior lines.
Across all participants, treatment-related adverse events (TRAEs) were generally mild to moderate, with no grade 4 or 5 toxicities reported. The most common grade 3 TRAEs included elevated levels of aspartate aminotransferase, aneemia and proteinuria.
The study is continuing with two dose expansion cohorts at 67.4 mg/m² every four weeks and 44.5 mg/m2 D1/D8 followed by 80 mg/m2 every four weeks starting at cycle 2.