Findings from the TROPiCS-02 trial show an overall survival benefit of sacituzumab govitecan in heavily pre-treated patients but raise questions about its optimal clinical implementation
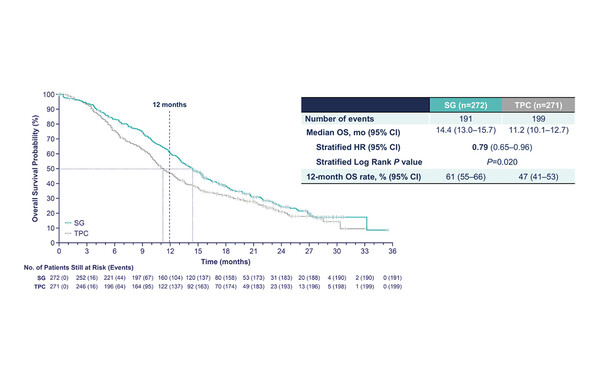
Data from the planned second interim overall survival (OS) analysis from the phase III TROPiCS-02 study of the antibody–drug conjugate (ADC) sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in heavily pre-treated patients with hormone receptor (HR)-positive/HER2-negative metastatic breast cancer were presented at ESMO Congress 2022 (LBA76). A statistically significant improvement in OS was observed with SG versus TPC (median 14.4 months versus 11.2 months; hazard ratio [HR] 0.79 [95% confidence interval (CI) 0.65–0.96]; p=0.02), along with significant improvements in objective response rate and quality of life (QoL) and manageable safety. TROPiCS-02 previously met its primary endpoint of statistically significant progression-free survival (PFS) benefit with SG (median 5.5 [95% CI 4.2–7.0] months; 12-month PFS rate 21%) versus TPC (median 4.0 [95% CI 3.1–4.4] months; 12-month PFS rate 7%) (J Clin Oncol. 2022:Aug 26).
“These data show that SG – targeting the Trop-2 molecule – is an extremely promising treatment option for those with heavily pre-treated metastatic breast cancer,” says Dr Giampaolo Bianchini, from Ospedale San Raffaele, Milan, Italy, commenting on the study. “We have effective endocrine therapies for HR-positive/HER2-negative patients which, for instance in combination with CDK4/6 inhibitors, have demonstrated a significant improvement in OS. However, when these therapies fail, we use chemotherapy and the efficacy of available agents decreases over subsequent treatment lines. Recently for patients with HER2-low tumours, trastuzumab deruxtecan demonstrated a remarkable clinical benefit; however, this does not impact patients with non-HER2-low tumours and those who are heavily pre-treated remain with limited treatment options and dismal outcome.”
Although the amount of benefit demonstrated is not transformative from a clinical practice perspective compared to the benefit demonstrated with the same agent in triple-negative breast cancer, according to Bianchini, results from the TROPiCS-02 trial show a clinically meaningful incremental impact for these patients with very limited treatment option that supports the inclusion of SG in the therapeutic algorithm. “However, some critical aspects need to be addressed for its optimal clinical implementation,” concludes Bianchini. “First, the efficacy of SG in this patient population after the use of trastuzumab deruxtecan in HER2-low tumours; second, the potential identification of biomarkers to identify patients who would benefit most from SG; and third, to consider these efficacy data in the context of QoL and patient-reported outcomes. The presentation at ESMO Congress 2022 showed a longer time to deterioration in EORTC QoL questionnaire (QLQ)-C30 global health scale/QoL (4.3 months versus 3.0 months; HR 0.75 [95% CI 0.61–0.92]; p=0.006) and fatigue (2.2 months versus 1.4 months; HR 0.73 [95% CI 0.60–0.89]; p=0.002) scales and further data would help us interpret the full magnitude of benefit of the ADC from the patient’s perspective.”
Abstract presented:
Rugo HS, et al. Overall survival (OS) results from the phase 3 TROPICS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (MBC) ESMO Congress 2022, LBA76
Proffered Paper Session – Breast cancer, metastatic, 09.09.2022, h. 16:00 – 17:30, Brest Auditorium