An interim analysis of phase III data further supports the use of abemaciclib plus non-steroidal aromatase inhibitor in patients with HR+, HER2– advanced breast cancer
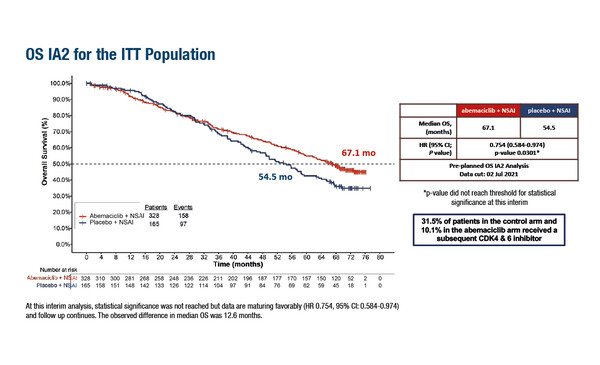
In a Late-Breaking Abstract presentation at ESMO Congress 2022, results from a pre-specified second interim analysis (IA2) of the phase III MONARCH 3 trial showed that the addition of abemaciclib to a non-steroidal aromatase inhibitor (NSAI) resulted in a 12.6-month increase in median overall survival (mOS) in patients with hormone receptor (HR)-positive, HER2-negative advanced breast cancer (LBA15).
Abemaciclib, a cyclin-dependent kinase 4 and 6 inhibitor, is approved in combination with an NSAI as initial therapy for post-menopausal patients with HR-positive, HER2-negative advanced breast cancer based on the significant improvement in progression-free survival – the primary endpoint – observed in the MONARCH 3 phase III trial (J Clin Oncol. 2017;35:3638–3646). OS is a key secondary endpoint in the study and a pre-specified IA2 was performed after ~252 events had occurred in the intent-to-treat (ITT) population, representing 80% of planned events for the final OS analysis.
MONARCH 3 is a randomised, double-blind trial conducted in 158 sites in 22 countries. Patients with HR-positive, HER2-negative recurrent breast cancer that was not amenable to surgical resection or radiotherapy with curative intent, or with metastatic disease were randomised 2:1 to receive an NSAI plus abemaciclib (n=328) or an NSAI plus placebo (n=165). At the time of IA2, the median follow-up was 5.8 years. In the ITT population, median OS was 67.1 months for abemaciclib plus NSAI compared with 54.5 months for placebo plus NSAI (hazard ratio [HR] 0.754; 95% confidence interval [CI] 0.584– 0.974; 2-sided p=0.0301). Similar findings were observed in the subgroup of patients with visceral disease (sVD, n=263) with median OS of 65.1 months for abemaciclib plus NSAI compared with 48.8 months for placebo plus NSAI (HR 0.708; 95% CI 0.508– 0.985; 2-sided p=0.0392). However, in both the ITT and sVD populations, the threshold for formal statistical significance was not met in the IA2 according to the pre-defined alpha spend procedure. Safety data were consistent with the known safety profile of abemaciclib. Follow-up for the patients enrolled in MONARCH 3 is ongoing and a final OS analysis is planned once at least 315 and 189 OS events have occurred in the ITT and sVD populations, respectively.
Abstract presented:
Goetz M, et al. MONARCH 3: Interim overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor in patients with HR+, HER2- advanced breast cancer. ESMO Congress 2022, LBA15
Proffered Paper Session, 09.09.2022, h. 16:00 – 17:30, Brest Auditorium