These results highlight the need to better investigate the mechanisms of action, define the optimal timing of administration and identify predictive biomarkers
At ESMO Congress 2022, several studies in patients with advanced breast cancer explored the efficacy of new endocrine therapies – a selective ER modulator (SERM) or different selective ER degraders (SERDs) – reporting mixed results. Notably, the SERDs studies confirm an important evolution from fulvestrant because these new therapies can be given orally.
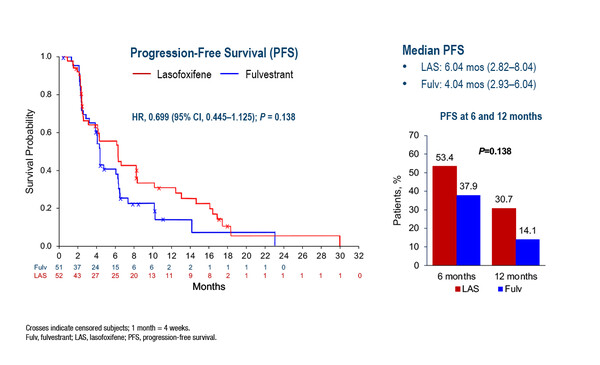
The first study, the open-label, phase II ELAINE 1 trial, evaluated the SERM lasofoxifene (5 mg/day orally) versus fulvestrant in patients with ER-positive/HER2-negative/ESR1-mutated advanced breast cancer who had progressed on aromatase inhibitors plus CDK4/6 inhibitors (LBA20). Median progression-free survival (PFS) – the primary endpoint – was 6.04 months with lasofoxifene versus 4.04 months with fulvestrant (hazard ratio [HR] 0.699; 95% confidence interval [CI] 0.445–1.125; p=0.138), which did not reach statistical significance. Other efficacy outcomes, including clinical benefit rate (36.5% versus 21.6%) and objective response rate (13.2% versus 2.9%), were numerically higher with lasofoxifene versus fulvestrant.
“Patients could only enter this trial if they had progression after 12 months, therefore,no patient had primary endocrine resistance,” says Dr Carmen Criscitiello from the European Institute of Oncology, Milan, Italy. “Although the clinical outcomes numerically favoured lasofoxifene over fulvestrant, these data are not sufficiently robust to confirm efficacy in this patient population and further studies are planned to clarify this. In addition, a comparison with a SERD in this context is warranted,” she adds.
Significant improvements in PFS with the SERD elacestrant (400 mg daily) versus endocrine therapy (fulvestrant or aromatase inhibitors) were reported in the phase III EMERALD trial in patients with ER-positive/HER2-negative advanced breast cancer who had received one or two prior endocrine therapies, mandatory pre-treatment with a CDK4/6 inhibitor and ≤1 prior line of chemotherapy (J Clin Oncol. 2022;May 18). Subgroup analysis of data from EMERALD (Abstract 220P) demonstrated prolonged PFS with elacestrant regardless of the type of endocrine therapy received as the physician’s choice of treatment (PCT) (HR 0.68; 95% CI 0.52–0.90 versus fulvestrant; HR 0.78; 95% CI 0.52–1.17 versus aromatase inhibitors). A similar trend was noted in patients with ESR1 mutations.
In a third study, the phase II acelERA trial, treatment with the SERD giredestrant (30 mg/day orally) was evaluated versus PCT (fulvestrant or aromatase inhibitors) in patients with ER-positive/HER2-negative advanced breast cancer who progressed after one or two lines of systemic therapy (prior fulvestrant and CDK4/6 inhibitor was allowed, as was ≤1 prior chemotherapy regimen and ≤1 targeted therapy) (Abstract 211MO). After a median follow-up of 7.89 months, the primary endpoint of investigator-assessed PFS was not met (HR 0.81; 95% CI 0.60–1.10; p=0.18). In total, 39% of patients had ESR1 mutations and the HR for PFS in these patients was 0.60 (95% CI 0.35–1.03).
Commenting on these data, Criscitiello says, “The latest results from the EMERALD and acelERA trials seem to be influenced by the different numbers and proportion of patients with ESR1 mutations in the trials (47.8% and 39%, respectively). Prior treatment with CDK4/6 inhibitors was not mandatory prior to treatment in the acelERA trial so patients in this trial may be less representative of a real-world patient population. For patients who derive a benefit, it will be interesting to see the long-term outcomes.”
Finally, negative results were reported from the phase II AMEERA-3 trial evaluating the SERD amcenestrant (400 mg/day orally) versus endocrine therapy of physician’s choice (fulvestrant, aromatase inhibitor, tamoxifen) in patients with endocrine-resistant ER-positive/HER2-negative advanced breast cancer (Abstract 212MO). Patients could have received ≤2 prior lines of endocrine therapy or ≤1 prior chemotherapy or ≤1 targeted therapy. The primary endpoint of PFS was not met, with median PFS of 3.6 months with amcenestrant versus 3.7 months with endocrine therapy (HR 1.051; 95% CI 0.789–1.4; p=0.6437).
“These results are disappointing,” admits Criscitiello, “as they do not show an efficacy benefit with amcenestrant. These data, along with interim data from the AMEERA-5 trial of combination palbociclib plus amcenestrant in first-line advanced breast cancer, have led to the decision by the company to terminate the development of this agent, including ongoing trials in the early setting. Data for the subgroup of patients with ESR1 mutation have not been provided, and the reason for this is unknown.”
While CDK4/6 inhibitors have defined a new standard of care in first-line treatment in both endocrine-sensitive and endocrine-resistant disease, research on new endocrine treatments will continue to play a role in the future as these agents contribute to delay the time to chemotherapy, overcome treatment resistance and improve patients’ quality of life when added to the treatment regimen.
According to Criscitiello, the latest advances in the field, such as those presented at ESMO Congress 2022 have shown that although ESR1 mutations represent a mechanism of acquired resistance, it is a potentially druggable mechanism and indeed, all these compounds displayed an increased sensitivity in patient harbouring this mutation. “Importantly, the role of SERDs in endocrine-sensitive disease, where ESR1 mutations are infrequent, is being investigated in two ongoing phase III trials – the persevERA trial of giredestrant plus palbociclib versus letrozole plus palbociclib (NCT04546009) and the SERENA-4 trial of camizestrant plus palbociclib versus anastrozole plus palbociclib (NCT04711252) – with results awaited with interest,” she says. “A pivotal outstanding question that must be addressed with further research before the treatment landscape can change is which patients will benefit the most,” Criscitiello concludes.
Abstracts presented:
Goetz M, et al. Open-label, randomized study of lasofoxifene (LAS) vs fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2- breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. ESMO Congress 2022, LBA20
Mini Oral Session – Breast cancer, metastatic, 10.09.2022, h. 14:45 – 16:15, Évry Auditorium
Martin M, et al. Giredestrant (GDC-9545) vs Physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2- locally advanced/metastatic breast cancer (LA/MBC): primary analysis of the phase 2 randomised, open-label acelera BC study. ESMO Congress 2022, Abstract 211MO
Mini Oral Session – Breast cancer, metastatic, 10.09.2022, h. 14:45 – 16:15, Évry Auditorium
Tolaney SM, et al. AMEERA-3, a phase 2 study of amcenestrant (AMC) versus endocrine treatment of physician’s choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2- advanced breast cancer (aBC). ESMO Congress 2022, Abstract 212MO
Mini Oral Session – Breast cancer, metastatic, 10.09.2022, h. 14:45 – 16:15, Évry Auditorium
Aftimos PG, et al. Elacestrant vs fulvestrant or aromatase inhibitor (AI) in phase 3 trial evaluating elacestrant, an oral selective estrogen receptor degrader (SERD), vs standard of care (SOC) endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC): subgroup analysis from EMERALD. ESMO Congress 2022, Abstract 220P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform