Numerically improved overall survival was shown with abemaciclib plus trastuzumab and fulvestrant in patients with advanced breast cancer
Final results from the monarcHER trial presented at ESMO Congress 2022 demonstrated numerically improved overall survival (OS) with abemaciclib, a selective cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor, in combination with HER2-targeted therapy (trastuzumab) with or without hormonal therapy (fulvestrant) compared with chemotherapy plus trastuzumab in patients with hormone receptor-positive, HER2-positive advanced breast cancer (LBA2806).
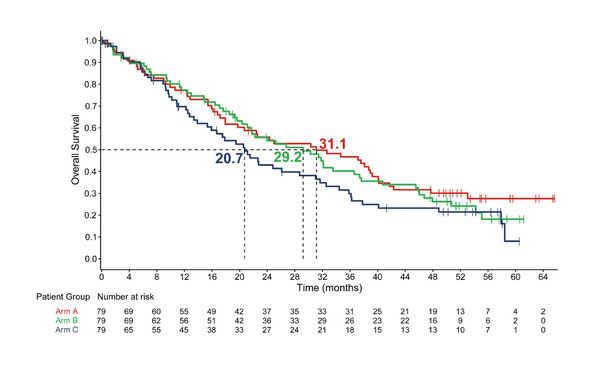
The open-label, phase II trial enrolled 237 patients with advanced breast cancer who had progressed on ≥2 anti-HER2 therapies. Patients were randomised 1:1:1 to one of three treatment arms: oral abemaciclib 150 mg bid, IV trastuzumab (8 mg/kg on cycle 1 followed by 6 mg/kg thereafter) and IM fulvestrant 500 mg (arm A); abemaciclib plus trastuzumab (arm B); or standard of-care single agent physician’s choice chemotherapy plus trastuzumab (arm C). After a median follow-up of 52.9 months (to end-March 2022), 157 deaths had occurred across the arms: 63%, 68% and 67% of patients in arms A, B and C, respectively. Median OS – a secondary outcome – was 31.1 months in arm A, 29.2 months in arm B and 20.7 months in arm C (arm A versus arm C: hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.47–1.21; p=0.243; arm B versus arm C: HR 0.73; 95% CI 0.46–1.15; p=0.177).
Previous analyses from this trial revealed that the primary endpoint was met, with significantly greater progression-free survival (PFS) in arm A than in arm C (8.3 months versus 5.7 months, respectively) (Lancet Oncol. 2020;21:763–775). Final PFS findings and safety outcomes were consistent with these earlier data.
An exploratory RNA sequencing analysis was performed to evaluate the impact of intrinsic breast cancer subtype on outcome and showed longer PFS in patients with luminal than non-luminal subtypes (8.6 months versus 5.4 months; HR 0.54; 95% CI 0.38–0.79). A similar trend was noted with OS data (31.7 months versus 19.7 months; HR 0.68; 95% CI 0.46–1.00).
These final data suggest that a triple-agent, chemotherapy-free treatment regimen may improve OS in patients with hormone receptor-positive, HER2-positive advanced breast cancer, a disease setting in which treatment options are limited.
Abstract presented:
F. André, et al. Final overall survival (OS) for abemaciclib plus trastuzumab +/- fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): a randomized, open-label, phase 2 trial. ESMO Congress 2022, Abstract 2806 LBA2806
Mini Oral session – 10.09.2022, 14:45 – 16:15, Évry Auditorium