AI technology is being tested across tumour types and with different purposes, with an emerging trend for adding value to whole slide image analyses for HER2 status
A number of presentations and posters at ESMO Congress 2022 show that artificial intelligence (AI) techniques are being employed in a myriad of applications in oncology with different aims, despite some limitations still restricting their adoption into clinical practice. An emerging trend is aiming to extract as much information as possible from data already available for the majority of patients with breast cancer – stained whole slide images – so as to gain potentially valuable insights into the disease.
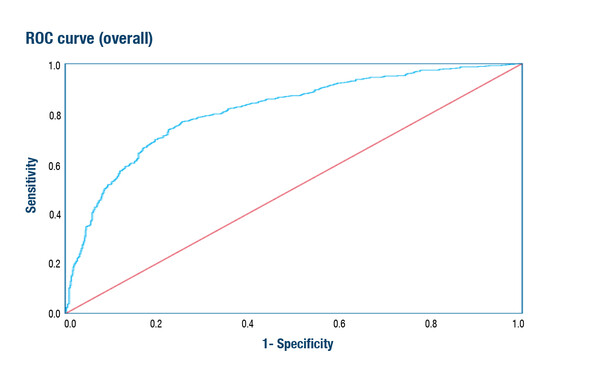
One presentation at this year’s Congress explored whether a machine learning model could be trained to identify HER2-positive tumours routinely from haematoxylin and eosin (H&E)-stained whole slide images (Abstract 68MO). The authors concluded that the model could predict with high accuracy the HER2 status of patients in a validation set; area under the receiver-operator curve (AUROC) of 0.81 (95% confidence interval [CI] 0.79–0.83) and balanced accuracy of 73.1%. When the model was generalised to assess slides derived from patients in a clinical study (GeparOcto), the AUROC was similar, at 0.80 (95% CI 0.77–0.83) while balanced accuracy was reduced to 70.4%.
A second study, which also evaluated a novel AI model developed to ‘read’ H&E-stained whole slide images, described how the model could successfully distinguish between HER2-negative and samples expressing low levels of HER2 (HER2-low) determined according to HER2 mRNA (Abstract 93P).
Commenting on these findings, Prof. Olivier Elemento from the Caryl and Israel Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY, USA, says, “The current gold standards for determining HER2 status are immunohistochemistry (IHC) and fluorescent in situ hybridisation (FISH). I do not think that current AI models applied to H&E slides can match the performance level of the gold standards for the determination of HER2 status and so I do not advocate the use of these AI models in current clinical practice.” Elemento also cautions that there is a great deal of between-centre variation in imaging results, which will impact on the reproducibility of AI model results, “Training a model at one centre will not necessarily be identical to training it at a different centre. It is necessary to demonstrate high accuracy across data from multiple centres, including centres that did not contribute data for model training.” Elemento also advocates that more emphasis is placed on AI model interpretability. He says, “AI models that mimic the process by which pathologists assess tissue, for example identifying individual cells, examining their morphology and staining patterns, or segmenting tissue images into different areas and examining their properties, could end up being more trusted and more likely to be adopted by clinical end-users compared to black-box approaches whose decision process is inscrutable.”
“HER2 testing provides a decisive tool for selecting targeted treatment options for patients with breast cancer and is part of routine assessment in those with newly diagnosed invasive disease and those with recurrent or metastatic tumours where biopsy tissue is available,” says Elemento, “Another key reason why HER2 status is especially interesting in recent times is because of the potential for antibody–drug conjugates (ADCs) to be of benefit to patients with HER2-low tumours. Having the ability to screen large databases of H&E-stained slides and identify patients with HER2-positive and HER2-low breast cancers may be particularly useful for selecting which groups of patients to enrol in clinical trials of ADCs and may eventually help guide treatment decisions.”
Another study presented at ESMO Congress 2022 described an AI-powered HER2 IHC analyser, used alongside a second AI-powered whole slide image analyser that identifies and quantifies tumour-infiltrating lymphocytes within the cancer epithelium or stroma from H&E-stained whole slide images (Abstract 155P). The analysers were used to evaluate whole slide images obtained from patients with stage I–III, HER2-positive breast cancer before and after HER2-targeted neoadjuvant chemotherapy, and test whether the techniques could overcome variations between pathologists when reading IHC staining results. The AI HER2 IHC analyser classified tumour cells by HER2 staining intensity and by HER2 IHC category according to the latest ASCO/CAP HER2 IHC evaluation algorithm. There was high (93.7%) concordance of HER2 categories between the results obtained using AI techniques and pathologists. AI-powered automated HER2 scoring and tumour-infiltrating lymphocyte analysis results aligned with pathological complete response to chemotherapy, suggesting that the techniques could be used to provide additional information for response predictions. “Many AI models recognise patterns within a tissue, but it is not always clear how the algorithms make their decisions – what specific features in the tissue images are recognised to make response predictions. Because of the high concordance between the algorithm results and the pathologist findings, it appears that the algorithms developed in this study are able to mimic what a trained pathologist recognises in tissue images. The pathologist can then understand the reasons behind AI-derived results, which is something that is lacking in many AI models that casts doubt among clinicians about their suitability for practice. This is particularly relevant and encouraging when considering the use of these particular algorithms in clinical practice, since we need to be highly confident that the results match those of a pathologist,” says Elemento.
The use of AI to predict treatment responses and prognosis in a range of cancers was also reported in other presentations at ESMO Congress 2022. Studies included the prediction of cisplatin-based neoadjuvant chemotherapy response in muscle-invasive bladder cancer (Abstract 1773P), relapse after treatment for localised colon cancer (Abstract 340P), prognosis of patients with high-grade serous ovarian carcinoma (Abstract 593P) and immunotherapy efficacy in patients with non-small-cell lung cancer (NSCLC), although further work is needed (Abstract 1071P). Machine learning methods also demonstrated heterogeneity within KRAS-mutated and KRAS G12C subtype in patients with NSCLC, which may help to support treatment individualisation (Abstract 1181P).
Figure.
Abstracts presented:
Hägele M, et al. Generalization of a deep learning model for HER2 status predictions on H&E-stained whole slide images derived from 3 neoadjuvant clinical studies. ESMO Congress 2022, Abstract 68MO
Mini Oral Session, 11.09.2022, h. 14:45 – 16:15, Dijon Auditorium
Goldfinger M, et al. An AI-driven computational biomarker from H&E slides recovers cases with low levels of HER2 from immunohistochemically HER2-negative breast cancers. ESMO Congress 2022, Abstract 93P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Cho SY, et al. Artificial Intelligence (AI) - powered human epidermal growth factor receptor-2 (HER2) and tumor-infiltrating lymphocytes (TIL) analysis for HER2-positive early breast cancer patients treated with HER2-targeted neoadjuvant chemotherapy (NAC). ESMO Congress 2022, Abstract 155P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Bhambhvani H, et al. Prediction of Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer: A Machine Learning Approach. ESMO Congress 2022, Abstract 1773P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Bueno Gomez A, et al. Prediction of relapse in colon cancer patients by machine learning models combining radiomics and deep features extracted from baseline Computed Tomography. ESMO Congress 2022, Abstract 340P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Kirkup C, et al. AI-powered analysis of nuclear morphology associated with prognosis in high-grade serous carcinoma. ESMO Congress 2022, Abstract 593P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Prelaj A, et al. Trustworthy Artificial Intelligence Models using Real World and circulating genomics data for the prediction of Immunotherapy efficacy in Non-Small Cell Lung Cancer patients. ESMO Congress 2022, Abstract 1071P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Loong HHF, et al. Heterogeneity and prognostic diagnosis of KRAS-mutated population and KRAS G12C subtype among patients (pts) with advanced NSCLC (aNSCLC): a real-world study aided by machine learning approaches. ESMO Congress 2022, Abstract 1181P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform