Although encouraging results are presented, standardisation of methodology and large-scale clinical trials are needed before potential candidates can be used in routine clinical practice
Translational studies presented at the ESMO Congress 2021 show that ctDNA reductions, T-cell receptor profiles, monocyte subsets and plasma protein profiling all have potential as biomarkers for selecting patients likely to benefit from immunotherapy.
The extent of ctDNA reduction would be a completely new way of assessing response to treatment and determining whether a patient should continue with treatment or not
There is a suggestion that early ctDNA reduction is a better surrogate of efficacy with the T-cell receptor (TCR)–anti-CD3 bispecific fusion protein, tebentafusp, than RECIST objective response, according to study results in patients with HLA-A*02:01-positive metastatic uveal melanoma (Abstract 1757O). ctDNA reduction at week 9 occurred in 70% of 99 patients with baseline measurements and the extent of ctDNA reduction was strongly associated with improved overall survival (R2=0.88; p<0.0001), even in patients with RECIST-defined progressive disease and stable disease. Prof. John Haanen from the Netherlands Cancer Institute, Amsterdam, thinks the data from this study may have interesting clinical implications. “The extent of ctDNA reduction would be a completely new way of assessing response to treatment and determining whether a patient should continue with treatment or not,” he says.
In another study, a circulating pre-treatment TCR repertoire was shown to be a potential biomarker for immune checkpoint inhibitors (ICIs) in 29 patients receiving pembrolizumab for non-small-cell lung cancer (NSCLC), 27 with PD-L1 ≥50% and 2 with unknown PD-L1 status (LBA68). Reduced number of unique clones and reduced Shannon diversity were associated with improved response rate to pembrolizumab (p=0.038 and p=0.021, respectively) and prolonged progression-free survival (hazard ratio [HR] 0.40; p=0.031 and HR 0.44, p=0.041, respectively). Reduced diversity evenness and increased clonality correlated with an increased risk of immune-related adverse events. Cautiously optimistic about the data, Haanen comments, “The findings are promising, but without results on the AUC ROC curve being 0.9 or higher, the method has some way to go before it could be applied in clinical practice.”
Results from a study using RNA sequencing of pre- and post-treatment peripheral CD14+ cells from patients with metastatic melanoma and paired healthy donors, confirm ICI-dependent modulation of circulating monocytes and identify potentially prognostic/predictive subsets (Abstract 1761MO). One such subset is a myeloid-derived suppressor cell signature that is negatively associated with development of autoimmune adverse events and that is modulated by combination ICIs.
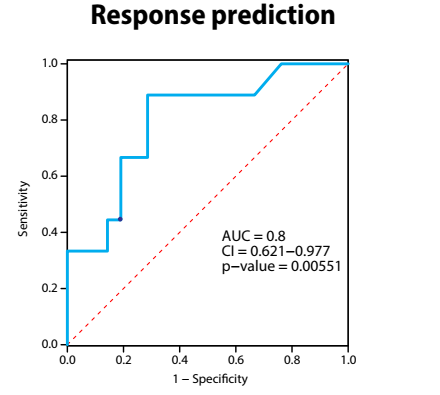
According to the results of a further study, a signature of eight proteins and two clinical parameters – identified using proteomic plasma profiling during ICI therapy in patients with NSCLC, coupled with machine learning and comprehensive clinical data – predicts outcome for ICI treatment (Abstract 74P). The validation set displayed an AUC ROC curve of 0.8 (p=0.006). Proteins and clinical parameters differed between responders and non-responders and ICI treatment induced pro-tumourigenic biological pathways in non-responders. “This is another good example of the use of novel technologies for mining blood samples for characteristics that may predict outcome to immunotherapy,” says Haanen. “The AUC ROC curve of around 0.8 is very promising but not yet good enough to allow the selection of patients for immunotherapy.”
The widespread use of these approaches will only be possible once we have harmonisation of protocols, from sample management to interpretation of results
Molecular pathologist, Prof. Umberto Malapelle from University Federico II of Naples, Italy, thinks there are barriers to be overcome before liquid biopsies can realise their full potential. “The goal is that liquid biopsy-based approaches – in association with tissue-based analysis – should enable us to characterise the mutational landscape of our patients’ tumours at baseline and during therapy,” he says. “The studies reported confirm that this method is key to routine clinical monitoring, regardless of the type of treatment. In particular, the study with tebentafusp clearly shows that liquid biopsy-based analysis, in contrast to RECIST criteria, was able to identify a subset of patients likely to have treatment response.” However, recognising that the pressing need for biomarkers predictive of response to ICIs has intensified the search, Malapelle warns that the utility of any liquid biopsy-identified markers will be limited if standardisation of techniques is not achieved. “The widespread use of these approaches in different laboratories and within different timeframes will only be possible once we have harmonisation of protocols, from sample management to interpretation of results,” he says. “This is one of the main challenges facing us.” Malapelle also talks of the need for large prospective trials to confirm early-phase study findings ahead of the routine availability of liquid biopsies for immunotherapy in the clinical setting. And he thinks that the future will involve a combined strategy. “It is likely that an approach integrating radiological evaluation and liquid-biopsy-determined 3D biology – DNA, RNA and protein analysis – will represent the best strategy for evaluating treatment response to immunotherapy.”
Haanen sums up the impact of the data for clinical practice. “Although these are early studies, the findings demonstrate the upcoming power of new technologies and biomarkers to tailor patient selection for treatment with immunotherapy,” he says, and concludes, “Machine-learning algorithms, together with larger datasets, will shape the way we use biomarkers in the future and will see us make huge progress in this field in the coming years.”
Shoushtari AN. Early reduction in ctDNA, regardless of best RECIST response, is associated with overall survival (OS) on tebentafusp in previously treated metastatic uveal melanoma (mUM) patients. ESMO Congress 2021, Abstract 1757O
Proffered Paper session - Translational research, Sun, 19.09.2021, h 13:30 - 14:50, Channel 5
Abed A. Clinical value of pre-treatment T-cell receptors (TCR) repertoire in non-small cell lung cancer (NSCLC) patients treated with single agent immunotherapy. ESMO Congress 2021, Abstract LBA68
Mini oral session - Translational research, Fri, 17.09.2021, h 17:30 - 18:30, Channel 7
Cooper R. Defining subset-wise myeloid responses to immune checkpoint blockade in melanoma. ESMO Congress 2021, Abstract 1761MO
Mini oral session - Translational research, Fri, 17.09.2021, h 17:30 - 18:30, Channel 7
Shaked Y. A predictive signature for response to immunotherapy in non-small cell lung cancer based on plasma proteomics and clinical parameters. Abstract ESMO Congress, Abstract 74P