Results from the ATALANTE-1 and MRTX-500 trials show efficacy improvements with immunotherapy in patients post-immune checkpoint inhibitor failure
Latest results from two randomised clinical trials presented at the ESMO Congress 2021 show exciting promise for new treatment options in patients with non-small-cell lung cancer (NSCLC) after failure on immunotherapy.
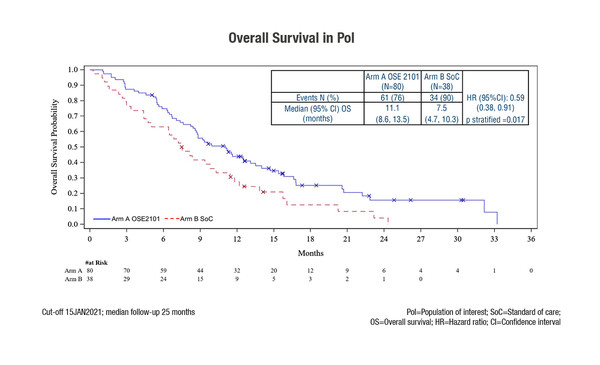
Final results were reported from the phase III randomised ATALANTE-1 trial, showing a median overall survival (OS) of 11.1 months with second- or third-line anticancer vaccine OSE-2101 and 7.5 months with standard-of-care docetaxel or pemetrexed (hazard ratio [HR] 0.59, 95% confidence interval [CI] 0.38–0.91; p=0.017) (LBA47). Median progression-free survival (PFS) was 2.7 months with OSE-2101 and 3.2 months with standard of care, while respective objective response rates (ORR) were 8% and 18%, and 6-month disease control rates were 25% and 24% (comparisons were non-significant). The study recruited patients with HLA-A2-positive and EGFR- and ALK-negative NSCLC who failed immune checkpoint inhibitor (ICI) therapy as last treatment. The study was stopped prematurely due to the risk posed by the COVID-19 pandemic on data integrity. As such, the final analyses were based on data from the population of interest (PoI) – those with failure after at least 12 weeks of immunotherapy in patients receiving sequential chemotherapy and immunotherapy. In total, the PoI included 118 (54%) of 219 patients enrolled in the study.
“These survival results are impressive and for the first time in a phase III setting, show improvements in patients with secondary resistance to immunotherapy, which is very encouraging. The clinically meaningful survival gain of 3.6 months is quite striking for this population of patients with a poor prognosis,” says Dr Antonio Calles from Hospital General Universitario Gregorio Marañón, Madrid, Spain.
However, Calles cautions that these are preliminary results applicable to a defined population of patients with HLA-A2-positive NSCLC previously exposed to immunotherapy. “The fact that the response rate, PFS and other clinical markers of response are similar, with no statistical differences compared with standard of care, may point to selection bias. At present, it is too early to change the standard of care, and the results needs to be confirmed in large studies.”
Updated efficacy data from the phase II MRTX-500 trial were also presented today, showing encouraging antitumour activity and OS results with the multiple kinase inhibitor sitravatinib in combination with the anti-PD-L1 antibody nivolumab in 68 patients with non-squamous NSCLC and failure of ICI therapy (anti-PD-1 or anti-PD-L1) and/or platinum chemotherapy (Abstract 1191O). Efficacy data for patients with prior clinical benefit from an ICI were analysed. After a median follow-up of 33.6 months, median OS was 14.9 months (95% CI 9.3–21.1), median PFS was 5.7 months (95% CI 4.9–7.6), ORR was 18% and median duration of response was 12.8 months. Two complete responses and 10 partial responses were reported. The most common grade 3–4 adverse events with sitravatinib plus nivolumab were hypertension and diarrhoea.
According to Calles, the study results are positive. “OS data with the combination of sitravatinib plus nivolumab are better than expected when considering historical control data, and this affirms that patients can be rechallenged with immunotherapy using this combination. Importantly, the adverse events with this combination are consistent with what is known from the mechanism of action of the combination against the tumour microenvironment and can be successfully managed with adequate treatment or dose reductions.”
Results from these two trials have identified patients that can derive benefit from further immunotherapy after prior failure, but larger confirmatory studies are required. The ongoing phase III SAPPHIRE study comparing sitravatinib plus nivolumab versus docetaxel in patients with advanced non-squamous NSCLC after disease progression on or after ICI therapy and platinum-based chemotherapy will hopefully confirm the risk/benefits seen with this combination in the MRTX-500 trial. “The focus of future research should aim to identify biomarkers to predict those patients most likely to respond and, in order to optimise tailored treatment for these patients, we also need a better understanding of the underlying mechanisms of resistance,” concludes Calles.
Besse B et al. Activity of OSE-2101 in HLA-A2+ non-small cell lung cancer (NSCLC) patients after failure to immune checkpoint inhibitors (IO): Final results of Phase 3 Atalante-1 randomised trial ESMO Virtual Congress 2021, LBA47
Proffered Paper session – NSCLC, metastatic 2, 20.9.2021, h. 13:30 – 13:40, Channel 4
Leal T A et al. MRTX-500: Phase 2 Trial of Sitravatinib (Sitra) + Nivolumab (Nivo) in Patients (Pts) With Nonsquamous (NSQ) Non–Small-Cell Lung Cancer (NSCLC) Progressing on or After Prior Checkpoint Inhibitor (CPI) Therapy. ESMO Virtual Congress 2021, 1191O
Proffered Paper session – NSCLC, metastatic 2, 20.9.2021, h. 14:20 – 14:30, Channel 4