Two phase I trials in patients with advanced EGFR-mutated NSCLC demonstrate promising potential of targeted therapy with amivantamab plus lazertinib
Encouraging preliminary findings from two ongoing studies of combination therapy with amivantamab plus the third-generation EGFR tyrosine kinase inhibitor (TKI) lazertinib were presented today at the ESMO Congress 2021, offering novel promise to a patient population that has a high unmet need for new treatments, particularly in later-line settings.
Amivantamab is a bispecific antibody that targets EGFR and c-mesenchymal epithelial transition factor (MET) receptors. The open-label phase I CHRYSALIS study is evaluating amivantamab both as monotherapy and in combination with lazertinib in 166 patients with EGFR-mutated NSCLC who have progressed on the EGFR TKI osimertinib (Abstract 1192MO). After a median follow up of 6.9 months, the objective response rate (ORR) in the monotherapy arm was 19% (95% confidence interval [CI], 12–27) and the clinical benefit rate (CBR) was 48% (95% CI, 39–57). Higher ORR and CBR rates were reported in the combination arm after a median follow-up of 11.1 months (36% [95% CI, 22–51] and 64% [95% CI, 49–78], respectively). Median duration of response in the monotherapy arm was 5.9 months and 9.6 months in the combination arm. Patients in the monotherapy arm were pre-selected for resistance mutations (including C797S) or MET amplification, whereas those in the combination arm were unselected and chemo-naïve.
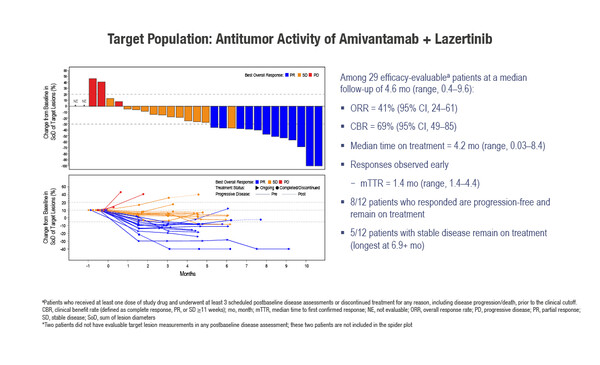
A second ongoing, phase I trial (CHRYSALIS-2) is examining amivantamab plus lazertinib in 136 patients with an EGFR Exon19 deletion or a L858R point mutation and disease progression after osimertinib and platinum chemotherapy (third-/fourth- or at least fifth line). Preliminary results show an ORR of 41% (95% CI, 24–61) among 29 patients evaluable for response at a median follow-up of 4.6 months, and 21% (95% CI, 11–36] in 47 heavily pre-treated patients (Abstract 1193MO).
In both CHRYSALIS trials, the safety profile of amivantamab as monotherapy or in combination with lazertinib was as expected and no new safety signals were noted.
Commenting on these data, Dr Anne-Marie Dingemans of Erasmus Medical Center, Rotterdam, the Netherlands, says, “These early efficacy findings from both studies – and the acceptable safety data – are encouraging. In the CHRYSALIS study, the higher ORR and CBR rates in the unselected combination-treatment group are perhaps unexpected, but nevertheless important, and highlight that we still need a better understanding of the mechanisms underlying these effects.”
As with all cancers we are striving for personalised medicine. Treatment decisions should preferably be based on the mutational profile and presence of resistance mechanisms in each individual patient.
However, caution is warranted. According to Dingemans, “The relatively low ORR rate in the heavily pre-treated patients in the CHRYSALIS-2 study suggests that this combination may not be practice-changing for this particular patient subset.” Tumour heterogeneity is a substantial challenge to the treatment of patients with EGFR-mutated advanced NSCLC, with heterogeneity likely to increase – even within the same patient – as the cancer progresses. “As with all cancers,” says Dingemans, “we are striving for personalised medicine. Treatment decisions should preferably be based on the mutational profile and presence of resistance mechanisms in each individual patient. It may therefore be prudent to re-biopsy the tumours and re-characterise their molecular profile each time a patient progresses on therapy.” Dingemans says that applying this approach in clinical practice is feasible, adding that, “Some of our patients have undergone up to four CT-guided tumour biopsies during their treatment. We should include this procedure, together with investigating liquid biopsies, whenever possible as this is the only way we can improve personalised treatment of these patients.”
“A particular challenge for patients with EGFR-mutated advanced NSCLC is that the duration of response decreases with later lines of treatment,” says Dingemans. “In addition, brain metastases become more problematic, with many patients suffering from symptomatic brain metastasis and/or radiation necrosis. Consequently, treatments with good brain penetration are needed.”
Both CHRYSALIS studies are ongoing and plan to enrol 780 and 520 participants, respectively. However, Dingemans think that comparative data and more detailed analyses of specific cohorts are needed. “While data from single-arm studies are very informative, randomised data are preferred, with stratification based on resistance mutations, line of treatment and the presence of brain metastases. In addition, biomarker-driven cohorts – according to resistance mechanism – are needed to understand which population of patients would gain additive value from this treatment,” concludes Dingemans.
Leighl NB et al. Amivantamab monotherapy and in combination with Lazertinib in post-osimertinib EGFR-mutant NSCLC: analysis from the CHRYSALIS study. ESMO Congress 2021, Abstract 1192MO
Mini oral session – NSCLC, metastatic 19.09.2021, h. 17:35 – 17:40, Channel 1
Shu CA et al. Amivantamab plus Lazertinib in post-osimertinib, post-platinum chemotherapy EGFR-mutant non-small cell lung cancer (NSCLC): preliminary results from CHRYSALIS-2. ESMO Congress 2021, Abstract 1193MO
Mini oral session – NSCLC, metastatic 19.09.2021, h. 17:40 – 17:45, Channel 1