Results show it may help to define more homogeneous subgroups of patients with grade II–III glioma for more timely and effective cancer care
WHO grade II–III gliomas are a biologically and prognostically heterogenous group, with survival ranging from less than a year to more than 10 years. A Mini Oral Session presentation today described an integrative analysis that may help in the development of multimodel prognostic models to improve stratification (Abstract 343MO).
Data from patients with histological diagnosis of WHO grade II–III glioma were classified into short-term survivors (overall survival [OS] <12 months after diagnosis; n=39) and long-term survivors (OS >10 years; n=123). Clinical features and DNA methylation patterns were then analysed in these two patient subgroups.
At diagnosis, Karnofsky Performance Scale (KPS) was lower (p<0.001) and age was higher (p<0.001) in short-term survivors as compared with long-term survivors. Furthermore, higher initial symptomatic burden was noted in short-term survivors, with more frequent motor deficits (p<0.001), aphasia (p=0.025) and visual disturbances (p=0.031) than in long-term survivors. However, epileptic seizures were more frequent in long-term survivors (p<0.001).
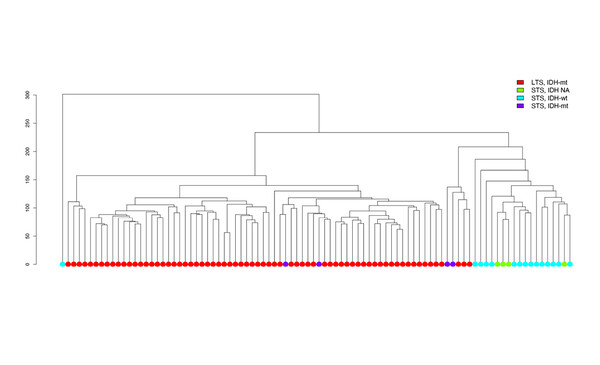
Unsupervised clustering of patient samples revealed a large cluster of patients who were long-term survivors with IDH-mutated tumours, a smaller cluster defined by short-term survivors with IDH-wild-type gliomas and a very small cluster of short-term survivors with IDH-mutated tumours.
Prof. Patrick Roth from the University Hospital and University of Zurich, Switzerland believes these results confirm and extend previous research in this topical area. “The findings of lower performance status and a high proportion of patients with wild-type IDH among short-term survivors are consistent with other work (Nat Rev Clin Oncol 2021;18:170–186; Acta Neuropathol 2014;128:561–571).” But he thinks that incorporation of DNA methylation profiling adds considerably to current methods of prognostication because DNA methylation technology allows clinicians to understand the classification and biological differences between gliomas to a much greater extent than is possible using pure histology or selected molecular markers. “Using DNA methylation analysis, the authors were able to identify distinct subgroups of tumours,” he explains. “Improved diagnostics would allow us to recognise much earlier if a tumour is likely to display aggressive characteristics and this could result in more comprehensive intensive treatment. On the other hand, it may also help us to avoid unnecessary and potentially harmful treatment in less malignant tumours that would currently be treated aggressively.”
Roth thinks use of DNA methylation profiling will become more widespread, particularly if this research can be repeated in a larger group of samples. “Over the next couple of years, it is likely that this technology will be expanded and improved so that it becomes an integral part of the diagnostic pathway of gliomas, particularly in large institutions and expert centres, where it will become a standard approach,” he concludes, adding a word of caution. “However, increasing segregation into smaller patient subgroups and the emergence of new tumour entities may make management more challenging for clinicians. The development of novel treatment strategies may not occur at the same pace as diagnostic procedures.”
Mair M J et al. Clinical features and DNA methylation patterns in long- and short-term survivors of WHO grade II-III glioma. ESMO Congress 2021, Abstract 343MO
Mini oral session, CNS tumours, 20.9.2021, h. 17:30 – 17:35, Channel 4