Results from early-phase studies reveal potential for a new cancer vaccine and adaptive immunotherapy
Three presentations at the ESMO Congress 2024 (Barcelona, 13–17 September) reported on therapies designed to stimulate or boost the body’s antitumour immune response in the management of aggressive, high-grade gliomas, which typically carry a poor prognosis.
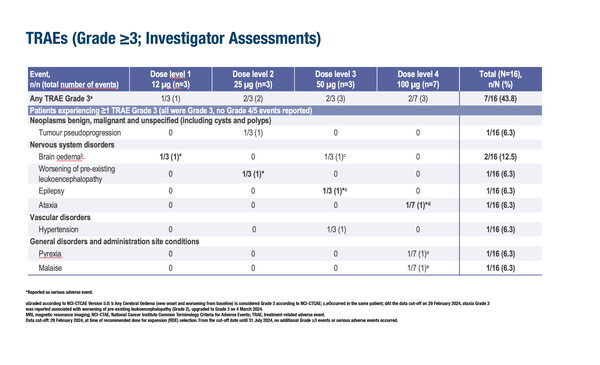
In a first-in-human study, intramuscular administration of the glioblastoma multiforme (GBM)-directed mRNA-based vaccine, CVGBM, was well tolerated during dose-escalation in patients with completely or partially resected MGMT-unmethylated grade 4 disease (Abstract 440O). All 16 patients treated completed the 2-week dose-limiting toxicity (DLT) evaluation period without DLTs. Only 6% of 156 treatment-emergent adverse events (TEAEs) were grade 3. Seven patients had nine grade ≥3 CVGBM-related AEs outside the DLT period, namely cerebral oedema (n=2) and worsening of pre-existing leucoencephalopathy, epilepsy, ataxia, hypertension, pyrexia and malaise (n=1 each). Tumour-associated antigen immune responses (CD4+ and/or CD8+) were reported in 77% of 13 evaluable patients, with 84% of these patients having T-cell responses primed and activated de novo by CVGBM. The dose-expansion part of the trial is ongoing (NCT05938387).
Another phase I trial, exploring the use of intracranial ipilimumab and nivolumab, reported that adding intracranial administration of autologous CD1c(BDCA-1)+ /CD141(BDCA-3)+ myeloid dendritic cells led to an encouraging 1-year overall survival rate of 60% in 21 patients with recurrent high-grade glioma (rHGG) (Abstract 441O). The median progression-free survival of 24 weeks was favourable compared with that reported for different cohorts with resectable rHGG in the study (NCT03233152). Treatment was discontinued early for disease progression in 9 patients and AEs in 3 patients. Among the most clinically important treatment-related AEs were bacterial colonisation of the Ommaya reservoir (n=3) and craniotomy wound dehiscence that became infected and caused bacterial meningitis (n=1). It is not clear whether the patients in this study had IDH mutations, which are now viewed as a disease entity that is different from IDH wild type tumours with a more favourable disease outcome (Br J Cancer. 2020;122:1580–1589). Also, there was no randomisation and results were compared to a historically pooled control cohort.
In the final study, intrathecal administration of allogeneic CAR ɣδT cells (QH104) to target B7H3, which is widely expressed in solid tumours, led to an objective response in 3 of 7 patients treated and disease control in all 7 patients with B7H3-positive recurrent GBM (Abstract 442O). In this dose-escalation trial, there were no DLTs. The most common AEs were fever (91.9%) and headache (14.5%), with an increase in interleukin-6 and interferon-ɣ in the CSF. No graft-versus-host disease was reported. QH104 showed persistence, being detectable in the CSF 30 days after administration. This phase I/II study is continuing (NCT06018363).
Programme details
Tabatabai G, et al. First in human study of the mRNA-based cancer vaccine CVGBM in patients (pts) with newly diagnosed and surgically resected MGMT-unmethylated glioblastoma (GBM): first results from the dose escalation phase. ESMO Congress 2024, Abstract 440O
Proffered Paper Session – CNS tumours, 13.09.2024, h. 14:00 – 15:30, Pamplona Auditorium – Hall 3
Neyns B, et al. A phase I clinical trial on the intracranial administration of autologous CD1c(BDCA-1)+ /CD141(BDCA-3)+ myeloid dendritic cells (myDC) in combination with ipilimumab (IPI) and nivolumab (NIVO) in patients with recurrent high-grade glioma (rHGG). ESMO Congress 2024, Abstract 441O
Proffered Paper Session – CNS tumours, 13.09.2024, h. 14:00 – 15:30, Pamplona Auditorium – Hall 3
Li X, et al. A phase I clinical trial of intrathecal injection of allogeneic CAR-γδT cells targeting B7H3 for the treatment of patients with recurrent glioblastoma. ESMO Congress 2024, Abstract 442O
Proffered Paper Session – CNS tumours, 13.09.2024, h. 14:00 – 15:30, Pamplona Auditorium – Hall 3