An exploratory analysis from the IMpower010 trial adds support to the use of post-chemotherapy adjuvant immunotherapy for selected early stage non-small-cell lung cancer
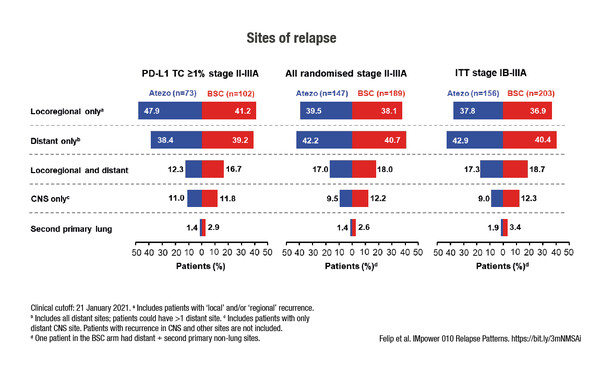
Among selected early stage non-small-cell lung cancer (NSCLC) patients with PD-L1 ≥1%, the relapse rate was lower with atezolizumab than with best supportive care (BSC) (29% versus 45%), according to results from an exploratory analysis of the phase III IMpower010 trial presented in today’s Presidential Symposium 3 (LBA9). No clear difference in the sites of relapse was observed between atezolizumab and BSC, with locoregional-only relapse occurring in 48% and 41% of patients, respectively, and distant-only relapse in 38% and 39%. Also, the use of post-relapse immunotherapy was more than three-times as high in the BSC arm (35%) compared with the atezolizumab arm (11%) and the use of other post-relapse treatment was similar between the arms.
These findings follow on from an interim analysis of the same trial, published earlier this year (J Clin Oncol 2021;39(15 Suppl):8500), which reported significant disease-free survival (DFS) benefits with the use of atezolizumab compared with BSC following adjuvant cisplatin-based chemotherapy, both in all randomised patients and in those with PD-L1 ≥1%. The risk of progression or death (hazard ratio [HR]) with atezolizumab versus BSC according to PD-L1 status was PD-L1 <1% HR 0.97 (95% confidence interval [CI] 0.72–1.31), PD-L1 1–49% HR 0.87 (95% CI 0.60–1.26) and PD-L1 ≥50% HR 0.43 (95% CI 0.27–0.68) among all randomised patients (n=882).
As expected, the use of immunotherapy was higher in the BSC arm than in the atezolizumab arm.
“The updated results from IMpower010 support the practice-changing outcomes from the interim DFS analysis and confirm the role of atezolizumab after adjuvant chemotherapy in patients with radically resected, early-stage NSCLC,” says Dr Antonio Passaro from the European Institute of Oncology, Milan, Italy. “It is reassuring that, although there was no clear difference in the pattern of relapse between the arms, the sites of relapse are consistent with what we would expect to see in this setting.” Regarding subsequent therapy use, Passaro comments: “As expected, the use of immunotherapy was higher in the BSC arm than in the atezolizumab arm. Long-term analysis of IMpower010 will be very useful for informing how we can use immunotherapy agents for metastatic disease after the use of atezolizumab in the adjuvant setting.”
Looking at the wider context, Passaro thinks that there needs to be a greater understanding of biomarkers for use in early-stage disease and a clearer definition of the optimum duration of adjuvant immunotherapy. These issues aside, he is very enthusiastic about the results: “These are very exciting times. Last year we had the report from the ADAURA trial (N Engl J Med 2020;383:1711-23), confirming the use of osimertinib in the adjuvant treatment of patients with EGFR-positive NSCLC. Now, with the IMpower010 study, we are seeing results that will really move immunotherapy into early-stage disease.”
Felip E et al. IMpower010: patterns of relapse and subsequent therapy from a Phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy (chemo) in stage IB-IIIA non-small cell lung cancer (NSCLC). ESMO Congress 2021, Abstract LBA9
Presidential symposium 3, 20.09.2021, h. 16:10 – 16:25, Channel 1
Simultaneous publication in The Lancet, DOI:https://doi.org/10.1016/S0140-6736(21)02098-5