Findings from three studies highlight that selecting patients for de-escalated treatment is still challenging
The not yet solved issue of optimal selection of patients with HER2-positive early breast cancer who may benefit most from de-escalation of treatment is the focus of three studies reported at the ESMO Congress 2021. “These abstracts summarise everything we have learned about HER2-positive disease over the past 10 years or so and re-confirm that it is possible to de-escalate treatment in some patients, which is very good news in this setting,” says Dr Evandro de Azambuja of the Jules Bordet Institute, Brussels, Belgium.
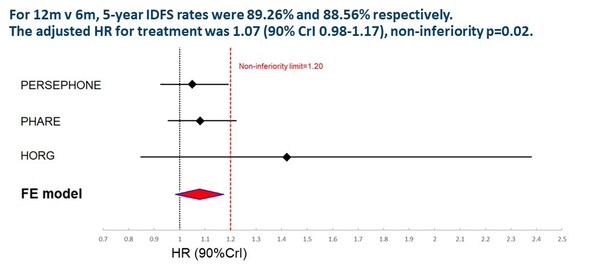
Two studies were discussed today in a Proffered Paper session. A meta-analysis of individual patient data from five randomised trials in patients with HER2-positive early breast cancer showed that 6-month treatment with the anti-HER2 antibody, trastuzumab, was non-inferior to 12-month treatment, with 5-year invasive disease-free survival (IDFS) rates of 88.6% and 89.3%, respectively (hazard ratio [HR] 1.07, 90% credibility interval 0.98–1.17; p=0.02) (LBA11) (Figure 1). The study was conducted to assess the non-inferiority of adjuvant trastuzumab given for less than 12 months compared with the standard duration of 12 months, with IDFS as the primary outcome. Trastuzumab treatment for 9 weeks did not meet the criteria for non-inferiority versus standard 12-month treatment.
Achievement of a pathological complete response (pCR) points to superior outcomes, potentially indicating that treatment de-escalation is possible, according to the results of a second meta-analysis (Abstract 117O). The study analysed survival data from four randomised studies of the tyrosine kinase inhibitor, lapatinib, in combination with neoadjuvant trastuzumab versus trastuzumab (plus anthracycline or paclitaxel chemotherapy) in patients with HER2-positive early breast cancer, and showed that pCR reduced the risk of relapse by 65% in patients with hormone receptor-negative tumours and by 40% in patients with hormone receptor-positive disease. In addition, rates of relapse-free survival (RFS) and overall survival were higher with lapatinib plus trastuzumab compared with trastuzumab (HR 0.62, 95% confidence interval 0.46–0.85 and HR 0.65, 95% CI 0.43–0.98, respectively).
Also, an analysis will be presented on 20 September revealing that gene expression of estrogen receptor 1 (ESR1) and CD8 were associated with improved IDFS, whereas clinical nodal burden was negatively associated with IDFS, according to results from the phase II WSG TP trial – part of the ADAPT umbrella (LBA13). The study is evaluating the predictive impact of biomarkers on outcomes after de-escalation of neoadjuvant treatment in patients with HER2-positive, hormone receptor-negative early breast cancer. Patients were randomised to neoadjuvant antibody–drug conjugate, trastuzumab emtansine (T-DM1), with or without endocrine therapy for 12 weeks versus trastuzumab plus endocrine therapy.
“Recent advances in the treatment of HER2-positive breast cancer have changed the prognosis for these patients. Where HER2 disease was once considered an aggressive disease, patients now have a very good prognosis,” comments de Azambuja. He is positive about the results presented at the ESMO Congress 2021, but cautions about the use of prognostic factors to guide treatment de-escalation. “We now need to identify biomarkers so we can define which patients can be de-escalated without compromising their prognosis. The neoadjuvant model is a good approach to both evaluate pCR with treatment and establish biomarkers. Although some biomarkers have been found, we are not at the stage where these can be used to select patients for treatment de-escalation. We know from the data presented here that pCR is a strong prognostic factor for patients with HER-2 positive breast cancer, but we also need to know why some of these patients relapse. Future research could include molecular imaging or circulating tumour DNA (ctDNA) monitoring to gain a better understanding of the disease process.” He adds, “I also think that survival outcomes are important endpoints to be studied in the neoadjuvant studies, while in the adjuvant setting, at the moment, there is no recommendation for the use of short trastuzumab duration.”
Looking to the future, de Azambuja concludes that, “Two interesting trials are currently exploring other de-escalation approaches. The CompassHER2-pCR trial is evaluating the impact of de-escalating chemotherapy in patients achieving pCR and escalating treatment in those with residual disease. Similarly, a second trial – the DECRESCENDO trial – will de-escalate adjuvant chemotherapy in patients with breast cancer, hormone receptor-negative and node-negative disease who achieve pCR after neoadjuvant chemotherapy and anti-HER2 blockade with pertuzumab and trastuzumab, and will escalate treatment in those with residual disease. These trials will hopefully further advance our understanding of this therapeutic strategy.”
Earl H. M. Individual patient data Meta-Analysis of 5 Non-Inferiority RCTs of Reduced Duration single agent adjuvant Trastuzumab in the treatment of HER2 positive Early Breast Cancer. ESMO Congress 2021, Abstract LBA11
Proffered Paper session – Breast cancer, early stage 17.9.2021, h. 13:30 – 13:40, Channel 5
Guarneri V. Survival after neoadjuvant therapy with trastuzumab-lapatinib and chemotherapy in patients with HER2-positive early breast cancer: a meta-analysis of randomised trials. ESMO Congress 2021, Abstract 117O
Proffered Paper session – Breast cancer, early stage 17.9.2021, h. 13:40 – 13:50, Channel 5
Harbeck N. Predictive impact of biomarkers on pCR and survival after de-escalated neoadjuvant T-DM1 with or without endocrine therapy (ET) vs. Trastuzumab+ET in HER2+/HR+ early breast cancer: WSG ADAPT TP trial. ESMO Congress 2021, Abstract LBA13
Mini Oral session – Breast cancer, early stage 20.9.2021, h. 17:43 – 17:48, Channel 1