Seemingly disappointing survival findings in the final results from the PEARL study are balanced by better quality of life with combination therapy
CDK4/6 inhibitors plus endocrine therapy (ET) appear to be the preferred alternative to chemotherapy for early treatment of hormone receptor (HR)-positive, HER2-negative metastatic breast cancer progressing on aromatase inhibitors (AIs). Although no survival benefit was observed in the final results of the phase III PEARL study (cohort 2) (Abstract 229MO), combination therapy seems to provide quality of life and tolerability benefits over chemotherapy.
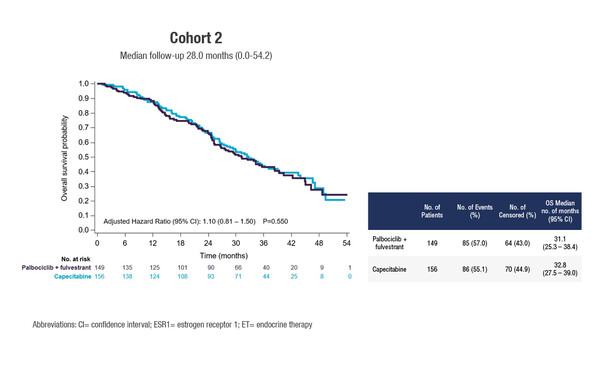
Median OS times of 31.1 months were reported with palbociclib plus fulvestrant (n=149) and 32.8 months with capecitabine (n=156) (adjusted hazard ratio 1.10; 95% confidence intervals 0.81–1.50; p=0.55) at a median follow-up of 28.0 months. The results follow earlier findings from the trial showing no progression-free survival (PFS) advantage for the combination over chemotherapy but an improved toxicity profile and a reduction in the time to deterioration of global health status.
Despite these apparently disappointing efficacy findings, Dr Carmen Criscitiello from the European Institute of Oncology, Milan, Italy, thinks that they provide important guidance for clinical decision -making. “The take-home message is that, even in the suboptimal setting of implementing ET plus CDK4/6 inhibitors, such as in the PEARL study, the combination of ET and CDK4/6 inhibitors improved quality of life compared with chemotherapy,” she says, adding, “We know that the ESMO Magnitude of Clinical Benefit Scale, for instance, regards the impact on quality of life as a significant consideration when assessing and grading clinical benefit of a new treatment.” In addition, a network meta-analysis has previously demonstrated that no chemotherapy regimen, alone or with targeted therapy, showed superior PFS to CDK4/6 inhibitors plus hormone therapy in the first- or second-line setting.
To better appreciate the implications of the PEARL study findings, they need to be placed in context with information from other studies. “At first glance, the efficacy results do not appear to compare well with those from previous studies,” Criscitiello notes. “For example, the second-line MONALEESA-3 (ribociclib and fulvestrant) and MONARCH-2 (abemaciclib and fulvestrant) trials showed PFS times longer than that achieved with palbociclib plus ET in the PEARL study. However, we know that resistance to CDK4/6 inhibitors is greater in advanced tumours and increases with the type and duration of treatment. Patients in cohort 2 of the PEARL study were more heavily pre-treated in the metastatic setting than those in the previous studies: around 27% received prior chemotherapy, 72% received prior AI and around 23% were receiving treatment in the third-line setting or beyond.” In contrast, in the phase II youngPEARL study – which included premenopausal women relapsing/progressing on tamoxifen who had received no prior AI for metastatic disease and only up to one line of chemotherapy – the combination of palbociclib plus exemestane was associated with a significant PFS benefit over capecitabine.
“CDK4/6 inhibitors plus ET remain the only real adequate option for the first-line treatment of HR-positive, HER2-negative MBC and have allowed our patients to live longer and to live better,” says Criscitiello. “Identifying the genomic landscape of the tumour to determine optimum sequencing of therapy and pinpoint predictive biomarkers may help to maximise the benefit of CDK4/6 inhibitors beyond first-line therapy.”
Jimenez M. M. Overall Survival (OS) of palbociclib (P) plus endocrine therapy (ET) versus capecitabine (CAP) in hormone-receptor+/HER2- metastatic breast cancer (MBC) that progressed on aromatase inhibitors (AIs). Final results of the PEARL study. ESMO Congress 2021, Abstract 229MO
Mini oral session – Breast cancer, metastatic, 18 September 2021, 17:35 – 17:40, Channel 1