Long-term outcomes of the phase III study confirm that only the addition of carboplatin to paclitaxel impacts on pathological complete response and event-free survival
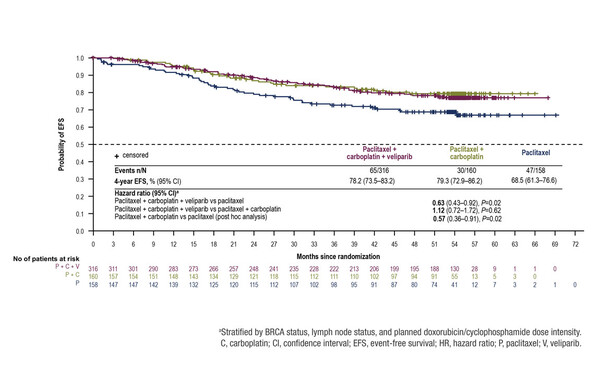
Results from analyses at a median 4.5 years of follow-up after surgery in the phase III BrighTNess study show that pathological complete response (pCR) and event-free survival (EFS) were improved in patients with triple-negative breast cancer (TNBC) with the addition of carboplatin plus paclitaxel to neoadjuvant chemotherapy, but not with the PARP inhibitor veliparib. The observed benefit was achieved without increasing the incidence of myelodysplastic syndrome or other secondary malignancies, such as acute leukaemia, chronic myeloid leukaemia and lung cancer. The long-term findings, which will be presented on Friday 17 September at the ESMO Congress 2021 (Abstract 119O), confirm the short-term results of the study published in 2018 (Figure).
All 634 patients randomised in the BrighTNess study had previously untreated stage II/III TNBC and the study was conducted at 145 centres worldwide. While the combination of carboplatin–paclitaxel–veliparib was associated with a significantly higher hazard ratio (HR) for EFS than paclitaxel alone (0.63; p=0.016), this triple combination was not superior to carboplatin–paclitaxel (HR 1.12; p=0.620). In a post-hoc analysis, the HR for EFS with carboplatin–paclitaxel versus paclitaxel was 0.57 (p=0.018).
Overall, the mortality rate across all treatment arms was low. The lowest incidence of death occurred in patients randomised to carboplatin plus paclitaxel (16/160, 10.0%), followed by carboplatin–paclitaxel–veliparib (38/316, 12.0%) and paclitaxel (22/158, 13.9%). While there were no statistical differences between treatment arms in relation to overall survival, the HR for overall survival was 18.0% lower with carboplatin–paclitaxel–veliparib than with paclitaxel (0.82; p=0.452) and 37.0% lower with carboplatin–paclitaxel than with paclitaxel (0.63; p=0.166). The triple combination was associated with a 25.0% higher HR for overall survival than carboplatin–paclitaxel (1.25; p=0.455).
One patient in each of the carboplatin–paclitaxel–veliparib (0.3%) and paclitaxel (0.6%) arms had myelodysplastic syndrome; there were none reported in the carboplatin–paclitaxel arm. Overall, secondary malignancies were slightly more common in the carboplatin–paclitaxel arm (6/158, 3.8%) than in the carboplatin–paclitaxel–veliparib (6/313, 1.9%) and paclitaxel (4/157, 2.5%) arms. Acute leukaemia, chronic myeloid leukaemia and lung cancer were absent in the carboplatin–paclitaxel and paclitaxel arms, and were each reported in one patient (0.3%) in the carboplatin–paclitaxel–veliparib arm.
Loibl S et al. Event-free survival (EFS), overall survival (OS), and safety of adding veliparib (V) plus carboplatin (Cb) or carboplatin alone to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) after ≥4 years of follow-up: BrighTNess, a randomized Phase 3 trial. ESMO Congress 2021, Abstract 119O
Proffered Paper session – Breast cancer, early stage 17.09.2021, h. 14:20 – 14:30, Channel 5