Significant progression-free survival benefits in pre-treated HER2-positive metastatic disease reported in the DESTINY-BREAST03 and SYD985.002/TULIP trials
Latest results from two phase III trials of antibody–drug conjugates targeting HER2 in patients with HER2-positive metastatic breast cancer may prove to be practice changing, as reported at the ESMO Congress 2021.
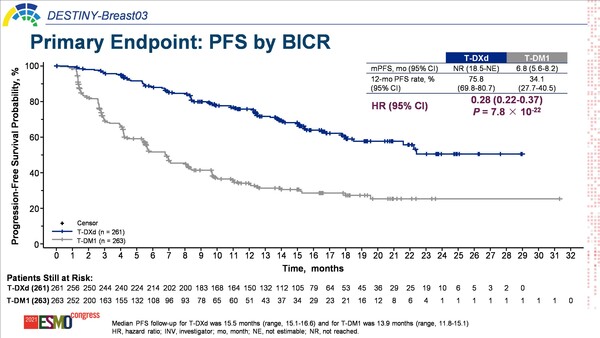
The open-label, randomised, phase III DESTINY-BREAST03 study compared trastuzumab deruxtecan (T-DXd) with trastuzumab emtansine (T-DM1), in patients previously treated with trastuzumab and a taxane. In results presented today, T-DXd significantly improved progression-free survival (PFS) – the primary endpoint – compared with T-DM1 (hazard ratio [HR] 0.28; p=7.8 × 10-22), with median PFS not yet reached and 6.8 months, respectively. Differences in estimated 12-month overall survival (OS) rates (94.1% with T-DXd versus 85.9% with T-DM1) did not cross the pre-specified boundary for significance (HR 0.56, 95% confidence interval [CI], 0.36–0.86) (LBA1).
According to Dr Aditya Bardia of Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA, the efficacy data are quite compelling. “It is wonderful to see such significant improvement in survival for patients with HER2-positive metastatic breast cancer, and the results will likely change practice, establishing T-DXd as the preferred therapy in the second-line setting.” Commenting on the early OS data, Bardia continues, “Although the 12-month OS rates are not significantly different, the median OS values are not yet estimable. Given the significant improvement in PFS, we would anticipate improvement in OS once the data maturity is sufficient, and we look forward to follow-up results.”
Results from these studies highlight the successful translation of science into development of potent therapies and will further increase interest in antibody–drug conjugates.
Preliminary data from a second phase III trial – the SYD985.002/TULIP trial – that compared [vic-]trastuzumab duocarmazine with physician’s choice of chemotherapy in patients who had received at least two prior lines of treatment, or previous treatment with T-DM1 will be presented tomorrow (LBA15). Median PFS by blinded central review – the primary endpoint – was 7.0 months for [vic-]trastuzumab duocarmazine and 4.9 months for chemotherapy (HR 0.64, 95% CI, 0.49–0.84; p=0.002). Bardia comments, “While this was a later-line trial than the DESTINY-BREAST03 trial, it is encouraging to observe clinically meaningful and potentially practice changing PFS improvements in patients receiving treatment in the third line and beyond. Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years – including T-DXd, neratinib, tucatinib, and margetuximab – and [vic-]trastuzumab duocarmazine could eventually be another option.”
In both studies, the antibody–drug conjugates were relatively well tolerated, though interstitial lung disease (ILD) requires early recognition and monitoring. Drug-related ILD/pneumonitis – an adverse event of interest based on data from the earlier phase II DESTINY-BREAST01 study – was reported in 10.5% of patients receiving T-DXd in the DESTINY-BREAST03 study, most (93%) of which were grade 1 or 2. In the SYD985.002/TULIP study, ILD/pneumonitis occurred in 7.6% of patients receiving [vic-]trastuzumab duocarmazine, including two grade 5 events.
“These phase III data are potentially practice changing for patients with metastatic breast cancer,” says Bardia, adding, “Results from both studies highlight the successful translation of science into development of potent therapies and will further increase interest in antibody–drug conjugates.” Bardia thinks that future research should explore the sequencing of these treatments in line with resistance, concluding that: “Further research needs to determine if there is any cross-resistance among these antibody–drug conjugates, which could impact their efficacy. Understanding the mechanism of resistance will have important implications for optimising antibody–drug conjugate sequencing for patients with breast cancer.”
Saura Manich C. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. ESMO Congress 2021, abstract LBA15
Proffered Paper session – Breast cancer, metastatic 19.9.2021, h. 13:30 – 13:40, Channel 1
Cortés J. Trastuzumab Deruxtecan (T-DXd) vs Trastuzumab Emtansine (T-DM1) in Patients (Pts) With HER2+ Metastatic Breast Cancer (mBC): Results of the Randomized Phase 3 DESTINY-Breast03 Study. ESMO Congress 2021, Abstract LBA1
Presidential symposium 1 – 18.9.2021, h. 15:05 – 15:20, Channel 1