Results from the PROMISE-meso trial presented
Treatment for relapsed malignant pleural mesothelioma (MPM) received a blow yesterday when eagerly awaited survival results of a phase III trial comparing pembrolizumab with chemotherapy failed to live up to their promise. The PROMISE-meso trial (Abstract LBA91_PR) is the first randomised trial to compare pembrolizumab and single-agent chemotherapy (gemcitabine or vinorelbine) in this setting, involving 144 patients.
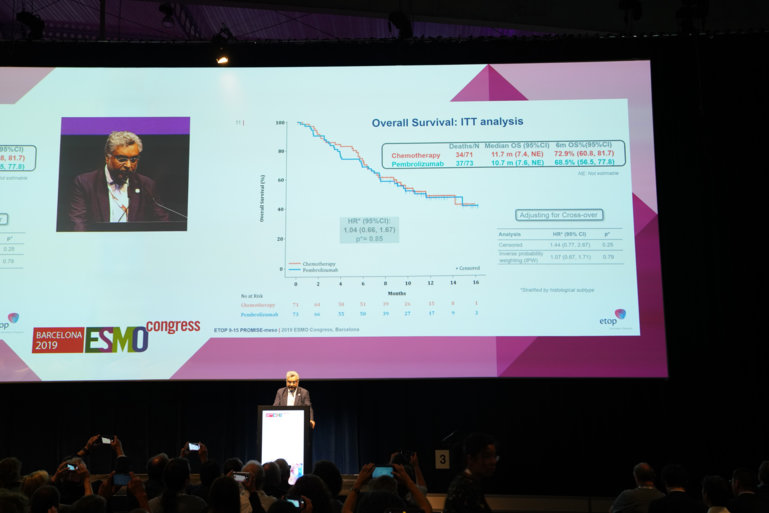
The presented results showed an overall response rate that was significantly higher with pembrolizumab (22% versus 6%, p=0.004) at a median follow-up of 12 months. However, there was no difference between pembrolizumab and chemotherapy in progression-free survival (median 2.5 months versus 3.4 months; hazard ratio [HR] 1.06; p=0.76) or overall survival (median 10.7 months versus 11.7 months; HR 1.05; p=0.85).
"The results from this trial are disappointing, but perhaps not surprising,” says Fennell. "The study faced two major challenges. Firstly, it compared pembrolizumab to a treatment—vinorelbine or gemcitabine—that is known to be active in relapsed MPM. Control arms are key in clinical trials. In the randomised CONFIRM trial1 we are conducting with nivolumab in relapsed MPM, we are using placebo as the control arm to give us the best chance of establishing the size of the treatment effect with the investigative agent.” The other major challenge for the PROMISE-meso trial, suggests Fennell, was that it enrolled an unselected patient population, which could have effectively diluted the magnitude of the pembrolizumab effect. But he thinks that this is not the end of the story. "There is likely to be a subgroup of patients, probably characterised by high tumour PD-L1 expression, who will benefit from pembrolizumab and this subgroup needs to be defined. Going forward, trials focussing on enriched patient populations may provide a greater understanding of the value of these treatments.”
"I do not think this is the end of the story. There is likely to be a subgroup of patients, probably characterised by high tumour PD-L1 expression, who will benefit from pembrolizumab and this subgroup needs to be defined,” says Prof. Dean Fennell from Leicester Cancer Research Centre, UK.Immunotherapy-related hyperprogression may represent a further potential complication with the use of checkpoint inhibitors in MPM. At ESMO Congress 2019, long-term results of a phase II study in relapsed MPM reported that single-agent nivolumab and a combination of nivolumab plus ipilimumab were associated with median long-term survival rates of 11.9 months and 15.9 months, respectively, with corresponding 2-year survival rates of 25.4% and 31.7% (Abstract 1841O). However, the 11 patients who were identified with hyperprogression measured according to tumour growth kinetics had worse survival (median 2.6 months) than patients with standard progression (median 5.5 months; HR 0.37; p=0.006) or patients with disease control (median 23.1 months; HR 0.12; p<0.0001).
It is hotly debated whether immunotherapy may lead to hyperprogression and evidence collected so far remains unclear. "We have treated a lot of patients with immunotherapy and we do not see hyperprogression,” comments Fennell. "It is unclear to us how it could arise and it would be really helpful to have a clear biological rationale. In my opinion, hyperprogression might be a very poor state of disease that is refractory to treatment. What we need are biomarkers to help us predict which patients have refractory disease so that we can protect them from receiving unnecessary immunotherapy.”Fennell believes that treatment with immunotherapy and targeted therapy is the way forward for relapsed MPM. This will involve both looking at different ways of using existing drugs, primarily in selected populations, and also exploring new drugs. Promising emerging therapies include tazemetostat in BAP1-inactive malignant mesothelioma, and arginosuccinate synthase 1.
The UK MiST trial is looking to accelerate the development of promising agents based on early signals of activity in patients prospectively stratified for treatment according to molecular screening."We have seen the quantum leap of what can be achieved with targeted therapies in lung cancer and I think that MPM should be no different,” he says. "The involvement of tumour suppressors, not oncogenes, makes the molecular targeting of genetic abnormalities in MPM more challenging, but it is achievable and there are some very encouraging developments down the road.”
ESMO Congress 2019 abstracts:
- LBA91_PR - A multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): Results from the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial
- 1841O - Second/third-line nivolumab vs nivo plus ipilimumab in malignant pleural mesothelioma: Long-term results of IFCT-1501 MAPS2 phase IIR trial with a focus on hyperprogression (HPD)