Efficacy and safety of the first-line novel combination were assessed in patients with untreated PD-L1 positive locally advanced or metastatic tumours
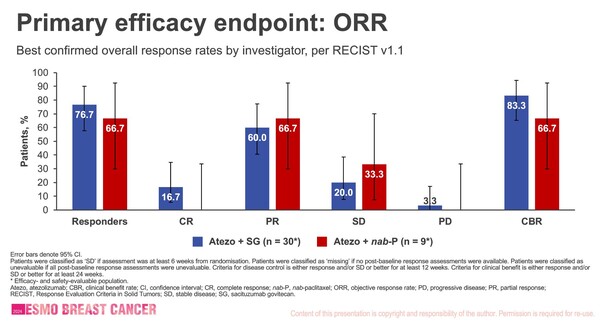
In an interim analysis presented at ESMO Breast Cancer 2024 (Berlin, 15–17 May), first line treatment with the combination of the PD-L1 inhibitor atezolizumab and sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate (ADC), showed a higher objective response rate compared to control (76.7% vs 66.7%) in patients with previously untreated PD-L1 positive locally advanced or metastatic triple-negative breast cancer (TNBC) (Abstract 181O).
The encouraging activity of the novel combination therapy was observed in a cohort of the MORPHEUS-panBC (NCT03424005) trial, an umbrella study evaluating the efficacy and safety of multiple treatment combinations in metastatic or inoperable locally advanced breast cancer.
At data cut-off (9 March 2023) a total of 42 patients were enrolled in the cohort 1 of the study and randomised to receive either the currently recommended first-line treatment with atezolizumab plus nab-paclitaxel (n=11) or atezolizumab plus sacituzumab govitecan (n=31) (atezolizumab 1200 mg IV D1 and sacituzumab govitecan 10 mg/kg IV D1 and 8; 21-D cycles). In the combination arm, five complete responses were observed (16.7%) while all partial responses were reported with the standard therapy. Clinical benefit rate (CBR) was 83.3% in patients treated with the novel combination therapy compared to 66.7% in the control arm.
Secondary endpoints data – i.e. progression-free survival (PFS) and duration of response (DOR) - were immature at the time of the analysis, however a trend towards a clinical benefit with atezolizumab combined with the ADC was reported compared to control (12.2 vs. 5.9 months; median follow-up: 10.6 vs. 11.7 months). A treatment benefit with the ADC was seen across all Trop-2 expression levels, with a trend towards higher response rates with higher Trop-2. Also, higher baseline stromal TILs trended with better response to atezolizumab plus the ADC. Safety of atezolizumab plus sacituzumab govitecan was consistent with the profiles of the individual agents.
Mature data of PFS and overall survival (OS) will clarify the potential synergic effect of immunotherapy with the ADC, and its clinical impact on patients.
Abstract discussed:
Schmid P., et al. Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: A Phase Ib/II study of multiple treatment (tx) combinations in pts with locally advanced/metastatic BC (LA/mBC). ESMO Breast Cancer 2024, Abstract 181O
Proffered Paper Session 1, 15.05.2024, h. 16:45 – 17:55, Berlin Hall