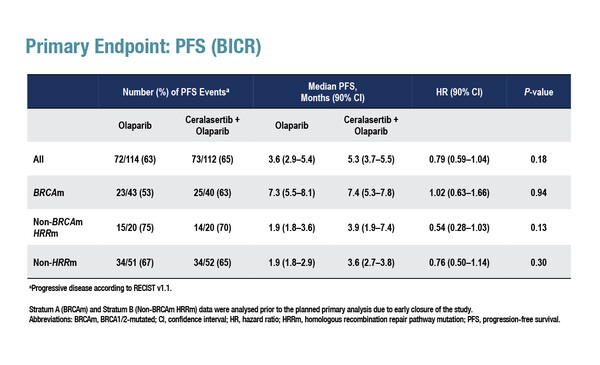
Results from the VIOLETTE trial also suggest that mutation status may influence response to each agent
Results from the phase II VIOLETTE trial presented at ESMO Breast Cancer 2022 report that among 273 patients with triple-negative breast cancer (TNBC), there was no significant difference in progression-free survival (PFS) – the primary endpoint – between olaparib plus ceralasertib and olaparib monotherapy, irrespective of tumour mutation status (Abstract 161O). The PFS hazard ratio (HR) was 1.02 (90% confidence interval [CI] 0.63–1.66; p=0.94) in patients with BRCA mutations (n=96), 0.54 (90% CI 0.28–1.03; p=0.13) in patients with homologous recombination repair (HRR) pathway mutations and no BRCA mutations (n=47) and 0.76 (90% CI 0.50–1.14; p=0.30) in patients without HRR pathway mutations (all-comers; n=130).
“Preclinical data and early clinical data have suggested potential synergism between PARP inhibitors and DNA damage response regulators, such as inhibitors of ataxia-telangiectasia mutated and Rad3-related (ATR) kinase, as well as WEE1,” says Dr Suzette Delaloge from Institut Gustave Roussy, Villejuif, France, an investigator on the trial, explaining its rationale. In the VIOLETTE trial, addition of ceralasertib to olaparib did not increase objective response rates (ORRs) compared with olaparib monotherapy in patients with BRCA mutations (50.0% versus 44.2%) or patients with HRR pathway mutations (20.0% versus 15.0%). However, the combination was associated with a greater ORR among patients without HRR pathway mutations (15.4% versus 3.9%) (odds ratio 4.45; 90% CI 1.30–21.20; p=0.04).
The most common adverse events (AEs) reported were anaemia and neutropenia. Grade ≥3 AEs were seen in 35.5% of patients receiving olaparib monotherapy and 46.8% of patients receiving the combination therapy.
The trial was stopped after the sponsor reviewed the efficacy data for patients with BRCA mutations, at which point the incomplete non-BRCAm HRRm stratum was underpowered to assess efficacy. A third arm of the trial, olaparib + the WEE1 inhibitor adavosertib, had previously been discontinued early due to greater than anticipated grade ≥3 haematological toxicity.
Delaloge agrees that the results are, on the whole, disappointing for the combination therapy, but thinks that there are some interesting observations that require further exploration. “Ceralasertib plus olaparib does not bring any benefit to patients with BRCA mutations but effects in the other two subgroups need clarification. Among patients with non-BRCA-mutated, HRR-mutated breast cancer and those with non-HRR-mutated breast cancer, the very low median PFS of 1.9 months with olaparib alone, compared with over 3 months for the combination, suggests that olaparib is not effective in these populations and that ATR inhibition alone could be having some effect,” she says. This is supported by the ORR results, at least in the all-comer population, which were higher among patients treated with the combination. “Together, these findings indicate that olaparib may be effective only in patients with metastatic breast cancer with a BRCA mutation. Conversely, there may be a level of activity of the ATR inhibitor alone in patients without BRCA mutations,” suggests Delaloge, noting that additional research is required to confirm these findings.
Abstract discussed:
Tutt A, et al. VIOLETTE: Randomised phase 2 study of olaparib (ola) + ceralasertib (cer) or adavosertib (ada) vs ola alone in patients (pts) with metastatic triple-negative breast cancer (mTNBC). ESMO Breast Cancer 2022, Abstract 161O
Proffered Paper Session 2, 04.05.2022, h. 16:45 – 18:15, Berlin Hall
Watch the session also on the Congress virtual platform.