In the first interim analysis of the ORIENT-31 trial, progression-free survival was slightly improved in patients who progressed after EGFR TKI, but mature results are needed to confirm whether combination therapy represents a new therapeutic option in this setting
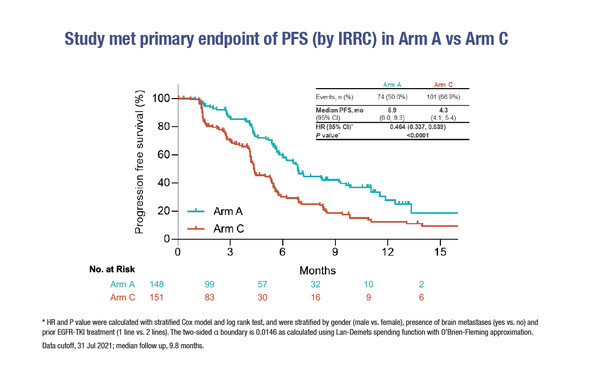
A presentation in an ESMO Virtual Plenary session during the ESMO Asia Virtual Oncology Week 2021 reports an encouraging, albeit small, improvement in progression-free survival (PFS) with sintilimab in combination with biosimilar bevacizumab plus chemotherapy (pemetrexed and cisplatin) compared with chemotherapy alone (hazard ratio [HR] 0.464; 95% confidence interval [CI] 0.337–0.639; p<0.0001) in patients with EGFR-mutated non-squamous non-small cell lung cancer (NSCLC) with prior EGFR-TKI treatment (Abstract VP9-2021).
In the double-blind phase III ORIENT-31 trial conducted in China, sintilimab plus chemotherapy showed a trend towards PFS benefit compared with chemotherapy alone (HR 0.750; 95% CI 0.555–1.013; p=0.0584), with data immature at the time of analysis. Median PFS was 6.9 months with sintilimab, bevacizumab plus chemotherapy, 5.6 months with sintilimab plus chemotherapy and 4.3 months with chemotherapy alone (Figure), while confirmed objective response rates were 43.9%, 33.1% and 25.2%, respectively. Grade ≥3 adverse events occurred in 54.7%, 39.3% and 51.0% of patients, respectively.
“Currently, the standard treatment for patients with EGFR-mutated NSCLC who progress with EGFR TKIs is platinum-based chemotherapy and there is a significant need for new therapeutic options. Different trials evaluating the use of immune checkpoint inhibitors as monotherapy have failed to show an improvement in efficacy endpoints in this setting,” says Dr Antonio Passaro, European Institute of Oncology, IRCCS, Milan, Italy, commenting on these results. Recently, the phase III IMpower150 trial showed an increased benefit with the investigational combination of atezolizumab, bevacizumab plus platinum-based chemotherapy in EGFR-mutated NSCLC; however, only data from sub-group analyses are available specifically in patients pre-treated with EGFR TKIs (J Thorac Oncol. 2021 Oct 6;S1556-0864(21)03217-2).
“The results from the ORIENT-31 trial are clearly positive in favour of sintilimab plus bevacizumab and chemotherapy but it is difficult to consider the small PFS improvement of less than 3 months as clinically significant. Mature results are eagerly awaited,” he adds.
Overall, one-third of patients with NSCLC harbour an EGFR mutation, with the highest prevalence observed in the Asian population (Oncotarget. 2016;7:78985–78993), thus making the need to validate new effective therapeutic options even more urgent in the Asia-Pacific region. Passaro thinks that more effort should be made to perform broad molecular characterisation of tumours progressing after first-, second- or third-generation EGFR TKIs to potentially identify the patients who could benefit the most from tumour-agnostic combinations, including VEGF inhibition and immunotherapy. “In this setting, there is a need to improve our understanding of EGFR heterogeneity and resistance mechanisms, and develop improved treatment strategies using new molecules, including sintilimab,” he concludes.
Lu S, et al. ORIENT-31: Phase III study of sintilimab with or without IBI305 plus chemotherapy in patients with EGFR mutated nonsquamous NSCLC who progressed after EGFR-TKI therapy. ESMO Asia Virtual Congress 2021, VP9-2021
ESMO Asia Virtual Congress 2021, Virtual Plenary, 19.11.2021, h. 19:10 – 19:25, Channel 1