However, regulatory and financial issues will shape when and where this happens
Recently presented practice-changing studies in early/localised non-small cell lung cancer (NSCLC) are likely to have a high impact in Asia. According to Prof. Tony Mok, Chinese University of Hong Kong, China, IMpower010 and DESTINY-Lung01 are at the top of the list of highlights, as discussed at yesterday’s ESMO Asia Highlights of the Year during the ESMO Asia Virtual Oncology Week 2021.
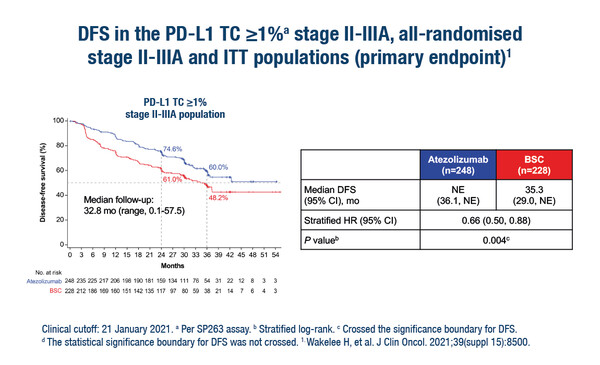
With primary results published recently in The Lancet (Lancet. 2021;398:1344–1357), and a sub-group analysis featured at the ESMO Virtual Congress 2021, IMpower010 reported that 1 year of adjuvant atezolizumab significantly improved disease-free survival (DFS) compared with best supportive care in patients with completely resected stage II–IIIA NSCLC (who had received adjuvant platinum-based chemotherapy) and tumour PD-L1 ≥1% (stratified hazard ratio [HR] 0.66; 95% confidence interval 0.50–0.88; p=0.004).
The cost of atezolizumab is unlikely to be reimbursed in most Asian countries so we need to better select the patients who are likely to benefit the most from the treatment.
“Despite a significant proportion of patients with resectable NSCLC and PD-L1 >1% will potentially benefit from these encouraging results, the cost of treatment is likely to drive an even more focused approach to treatment,” explains Mok.
“The cost of atezolizumab is unlikely to be reimbursed in most Asian countries so we need to better select the patients who are likely to benefit the most from the treatment. Sub-group analyses of IMpower010 showed that the DFS advantage of atezolizumab was greater in patients with PD-L1 >50% (HR 0.43) compared with those with PD-L1 1–49% (HR of 0.87). On the basis of these results, it is likely that in Asia we will refine the target to patients who have PD-L1 >50%.”
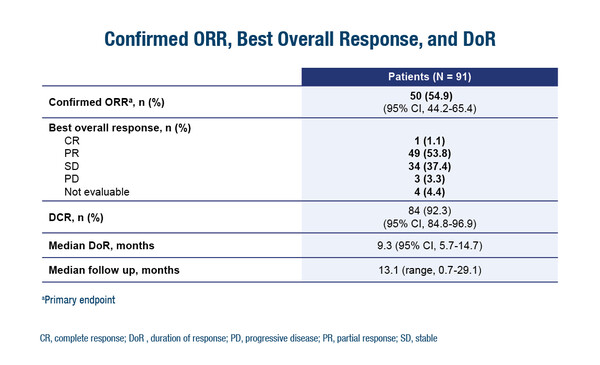
More obstacles are foreseen to the practice-changing impact of the DESTINY-Lung01 study in the Asian region. This phase II study investigated the HER2 antibody–drug conjugate trastuzumab deruxtecan in patients with metastatic HER2-mutated NSCLC refractory to standard treatment, showing an objective response rate of 55% and a median progression-free survival time of 8.2 months (N Engl J Med. 2021;Sep 18:epub ahead of print).
“While the impact of IMpower010 will be helped by the fact that atezolizumab already has US FDA approval, trastuzumab deruxtecan was granted breakthrough therapy designation by the FDA earlier this year, so its effects on Asian practice will take longer to be seen,” explains Mok. Added to this is the time needed to incorporate results into clinical practice guidelines and to effect healthcare policy changes across the region.
All of these factors will be affected by the considerable variations in healthcare systems, budgets and reimbursement arrangements across countries in Asia. “Longer-term data confirming the true benefit of treatment may help to shape reimbursement policies,” concludes Mok. “Until then, only the countries with fewer financial constraints – or whose patients can pay for their treatments – will likely adopt these novel treatment approaches in the short term.”
Mok T. Practice changing studies in localized/early disease. ESMO Asia Virtual Oncology Week 2021
Current standards and practice changing studies in thoracic cancers, ESMO Asia Virtual Oncology Week 2021, 19.11.21, h. 14:00 – 14:20, Channel 2