Promising results support the further development of new small-molecule PD-L1 inhibitors with favourable benefit–risk profiles and improved ease of use compared with antibodies
Early-phase Chinese trials presented at the ESMO Asia Congress 2024 (Singapore, 6–8 December) report the potential for two new oral small-molecule immune checkpoint inhibitors (ICIs) in solid tumours.
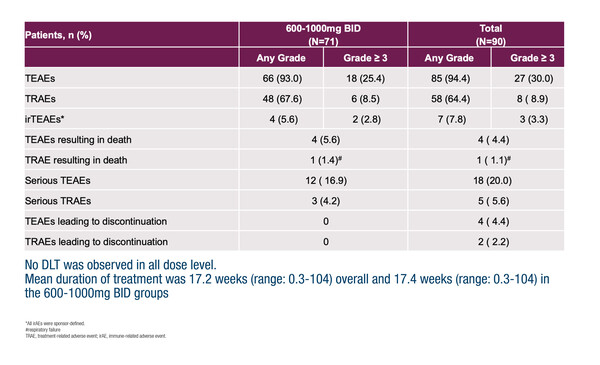
Latest findings from the first-in-human phase I trial with the oral, small-molecule PD-L1 inhibitor, ABSK043, showed a favourable safety profile and encouraging preliminary anti-tumour activity, particularly in non-small cell lung cancer (NSCLC) with high PD-L1 expression (Abstract 478O), both consistent with the experience with antibody ICIs. Grade ≥3 treatment-related adverse events (TRAEs) occurred in 6 out of 71 patients (8.5%) who received pharmacologically active doses of ABSK043 (600–1,000 mg bid), most commonly anaemia (3 patients), which did not lead to treatment discontinuation. No dose-limiting toxicities were observed and no peripheral neuropathy was reported.
The objective response rate (ORR) was 19.6% in 51 evaluable patients who received ABSK043 600–1,000 mg bid, of whom 49 were ICI naïve. Notably, the ORR was 41.7% (5 partial responses [PRs]) in 12 ICI-naïve patients with NSCLC who had a PD-L1 tumour proportion score ≥50%, which included patients whose disease carried EGFR mutations. Small-molecule PD-L1 inhibitors dimerise PD-L1 to cause down-regulation of the receptor and a dose-dependent decrease in PD-L1 expression was observed, alongside T-cell and cytokine changes. Expansions are planned for NSCLC and microsatellite instability-high/mismatch repair deficient tumours.
Manageable safety, favourable pharmacokinetics and promising antitumour activity were observed in an ongoing first-in-human phase I trial of BPI-371153, which also induces PD-L1 dimerisation and internalisation to disrupt the PD-1/PD-L1 interaction (Abstract 59O). Twenty patients received at least one dose of BPI-371153 (100–1,600 mg qd): 16 patients with solid tumours (11 with NSCLC) and 4 with recurrent/refractory lymphomas. No dose-limiting toxicities were reported and the maximum tolerated dose was not reached. Grade ≥3 TRAEs were reported in 3 patients (diarrhoea [600 mg qd], lymphopenia and skin infection [both 1,600 mg qd]) and did not lead to treatment discontinuation. There were no treatment-related deaths and peripheral neuropathy was not reported. BPI-371153 had a half-life of 20.8 to 26.2 hours. In pharmacodynamic analyses, increased IL-18, IL-2, IL-6, CXCL-9 and soluble PD-L1 levels were observed, indicating immunomodulatory effects. Notably, the ORR was 23.5% with 1 complete response and 3 PRs in patients who had high PD-L1 expression or high tumour mutational burden. BPI-371153 1,200 mg qd has been selected for the expansion phase.
Oral ICIs offer tangible benefits over their antibody counterparts. “Small-molecule ICIs are cheaper to manufacture and deliver, may be more convenient for patients, are not associated with infusion reactions, are expected to have better penetration into tumours and may have advantages in the management of immune-related adverse events due to shorter half-lives,” says Dr Stefan Symeonides from the Edinburgh Cancer Research Centre, University of Edinburgh, UK. However, these agents will need to match the efficacy of those antibody counterparts, while not introducing any significant new risks, and development has not been straightforward.
“From these phase I results, it appears that ABSK043 and BPI-371153 have similar tolerability profiles to ICI antibodies, with no peripheral neuropathy, and with some clear evidence of antitumour activity, supported by correlative pharmacodynamic data,” observes Symeonides. He thinks that further trials with these small molecules are warranted and timely, particularly in view of the recent discontinuation of other agents. He notes that other agents including OX-4224 (evixapodlin/GS-4224), AB-101 and ASC61 are also undergoing early testing. “It could be that the development of oral ICIs is not thought to be financially attractive in some markets, but I can see the strong appeal of having affordable oral ICIs if they deliver on the benefit–risk profile. There is a gap in the market now, but if the development of oral ICIs takes too long, PD-L1 inhibitors – antibodies and small molecules – may be overtaken by potential next-generation immunotherapies, such as the anti-PD-L1/VEGF bispecific antibodies, which are also moving forward in this space,” he concludes.
Programme details:
Yu Y, et al. Updated results of oral PD-L1 inhibitor ABSK043 in advanced solid tumor patients (pts) from a phase I study. ESMO Asia Congress 2024, Abstract 478O
Proffered Paper Session: Developmental and Precision Medicine, 06.12.2024, h. 10:15 – 11:45, Hall 402
Yang J, et al. A first-in-human, phase I study of BPI-371153, a novel small-molecule PD-L1 inhibitor, in advanced solid tumors or recurrent/refractory (r/r) lymphoma. ESMO Asia Congress 2024, Abstract 59O
Proffered Paper Session: Developmental and Precision Medicine, 06.12.2024, h. 10:15 – 11:45, Hall 402