CheckMate 722 trial fails to meet its primary endpoint in patients with cancer progressing on one or two prior lines of EGFR tyrosine-kinase inhibitors
A presentation at the Presidential Symposium at ESMO Asia Congress 2022 (Singapore, 2–4 December) reports that adding a checkpoint inhibitor to chemotherapy does not improve outcome in patients with non-small-cell lung cancer (NSCLC) progressing on EGFR tyrosine kinase inhibitors (TKIs) (LBA8). Data from the final analysis of the CheckMate 722 trial were eagerly awaited as it is the first randomised phase III trial to evaluate the use of immunotherapy in addition to standard platinum chemotherapy in patients with disease harbouring EGFR mutations (common and uncommon) and resistant to EGFR TKIs.
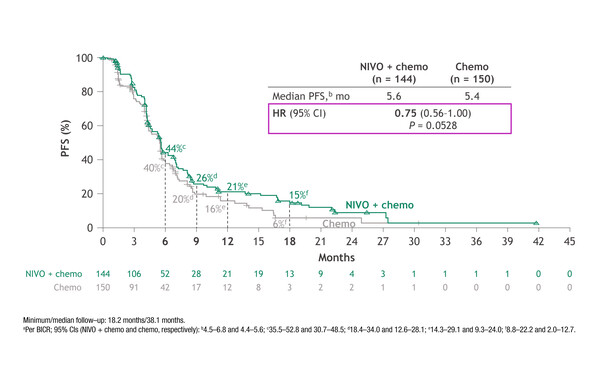
No significant improvement in median progression-free survival (PFS) with nivolumab plus platinum and pemetrexed compared with chemotherapy alone was reported in 294 patients with EGFR-mutated NSCLC progressing on one or two prior lines of TKIs. At a minimum follow-up of 18.2 months, PFS was 5.6 months (95% confidence interval [CI] 4.5–6.8) with the combination and 5.4 months (95% CI 4.4–5.6) with chemotherapy alone (hazard ratio [HR] 0.75; 95% CI 0.56–1.00; p=0.053). The PFS findings were generally consistent across subgroups, with a non-significant trend (5.6 versus 5.4 months; HR 0.72; 95% CI 0.54–0.97) in favour of patients with common sensitising EGFR mutations (269/294) and those with one prior line of EGFR TKI treatment (248/294).
The median overall survival (OS) was 19.4 months (95% CI 16.1–21.0) with nivolumab plus chemotherapy and 15.9 months (95% CI 14.0–18.8) with chemotherapy alone (HR 0.82; 95% CI 0.61–1.10). Objective response rates (ORRs) were 31% with nivolumab plus chemotherapy and 27% with chemotherapy alone, with corresponding median duration of response (DoR) times of 6.7 months and 5.6 months. Grade 3–4 treatment-related adverse events were reported in 45% of patients receiving nivolumab-based therapy and 29% of those receiving chemotherapy alone.
“The use of immune checkpoint inhibitors showed limited improvement in EGFR-mutant disease, naïve or resistant to standard treatment, based on the low immunogenicity of this particular molecular-driven lung cancer. With the background of positive results from the IMpower150 (N Engl J Med. 2018;378:2288–2301) and ORIENT-31 (Lancet Oncol. 2022;23:1167–1179) trials investigating chemotherapy and immunotherapy – plus anti-angiogenetic agents – in this setting, there were great expectations for the CheckMate 722 trial focused specifically on EGFR-mutated disease, so these findings might be considered quite disappointing,” says Dr Antonio Passaro from the European Institute of Oncology, Milan, Italy. However, he thinks that limited clinical benefit may have been expected in resistant patients previously exposed to EGFR TKIs due to the diversity of mechanisms of resistance and related immunogenic profiles, which currently remain controversial and should be further elucidated (e.g. on-target alterations versus off-target alterations).
Passaro notes that the trend in favour of patients with common sensitising EGFR mutations and in those treated with only one prior EGFR TKI are difficult to fully interpret considering that the study results were statistically underpowered due to the sample size reduction and the heterogeneity of the population. In addition, only a small proportion of enrolled patients received osimertinib as the last line of treatment. “To improve our understanding, there should be further evaluation of tissue (archival and after EGFR TKI therapy) to identify the molecularly driven subgroups that may be potentially sensitive to an immune-based combination in the resistance setting,” he comments.
Also presented at the Presidential Symposium, a second phase III trial reported that the TKI lazertinib might be a valid alternative to osimertinib as a first-line treatment for EGFR-mutated advanced NSCLC.
Lazertinib significantly improved the primary endpoint (PFS) compared with gefitinib (median 20.6 months versus 9.7 months; HR 0.45; 95% 0.34–0.58; p<0.001) in the interim analysis of the LASER301 trial, which is investigating the first-line treatment of 393 patients with EGFR-mutated advanced NSCLC (LBA7).The PFS benefit of lazertinib over gefitinib was consistent across all predefined subgroups, including Asian (20.6 months versus 9.7 months) and non-Asian (not reached versus 9.7 months) patients. An ORR of 76% was observed in both treatment arms, with a median DoR of 19.4 months (95% CI 16.6–24.9) with lazertinib and 8.3 months (95% CI 6.9–10.9) with gefitinib. OS data were immature, but OS rates at 18 months were 80% with lazertinib and 72% with gefitinib. The safety profiles of lazertinib and gefitinib were in line with those previously reported.
“The results from the LASER301 trial reflect the PFS benefits shown with third-generation EGFR TKIs over first-generation TKIs in the first-line treatment of EGFR-mutated NSCLC in the FLAURA (osimertinib, N Engl J Med. 2018;378:113–125) and AENEAS (aumolertinib, J Clin Oncol. 2022;40:3162–3171) trials,” says Passaro. “The confirmation that lazertinib improved outcomes similarly across Asian and non-Asian populations is particularly important given that the trial could be the driver for lazertinib approval in Asian countries where osimertinib is not yet approved or reimbursed,” he concludes.
Abstracts discussed:
Mok TSK, et al. Nivolumab (NIVO) + chemotherapy vs chemotherapy in patients (pts) with EGFR-mutated metastatic non-small cell lung cancer (mNSCLC) with disease progression after EGFR tyrosine kinase inhibitors (TKIs) in CheckMate 722. ESMO Asia Congress 2022, LBA8
Presidential Symposium 03.12.2022, h. 10:45 – 12:15, Hall 406. Also watch the session on the Congress virtual platform.
Cho BC, et al. A randomized, double-blind, multinational phase III study to assess the efficacy and safety of lazertinib versus gefitinib in the first-line treatment of patients with EGFR mutation (EGFRm), advanced NSCLC (LASER301; NCT04248829). ESMO Asia Congress 2022, LBA7
Presidential Symposium 03.12.2022, h. 10:45 – 12:15, Hall 406. Also watch the session on the Congress virtual platform.