Leveraging artificial intelligence and digital pathology, oncology is entering a transformative stage where advanced image analysis and AI-driven biomarkers enable personalised cancer treatment decisions.
Author: Torbjørn Furuseth, MD, CEO & Co-founder of DoMore Diagnostics
The content of this article was provided by DoMore Diagnostics and reflects the views of the author. ESMO does not endorse the content of this publication and does not take any responsibility for the accuracy, completeness or reliability of the information contained in such article.
Artificial intelligence (AI) is poised to transform oncology in many aspects, particularly through powerful image analysis capabilities that decode complex molecular profiles, tissue morphology, and progression trajectories. From digitised slides of standard pathology stains, AI can decipher important information “hidden” in the tumour tissue architecture and cellular structure and even predict patient outcomes, thus supporting oncologists to personalise treatment decisions with even greater precision.
Surgery is the cornerstone of treatment for locally advanced cancer, curing most patients. However, the decision for adjuvant therapy after surgery is difficult. The lack of robust prognostic tools leads to significant overtreatment of patients, exposing patients to unnecessary toxicity, reduced quality of life, and considerable healthcare costs.
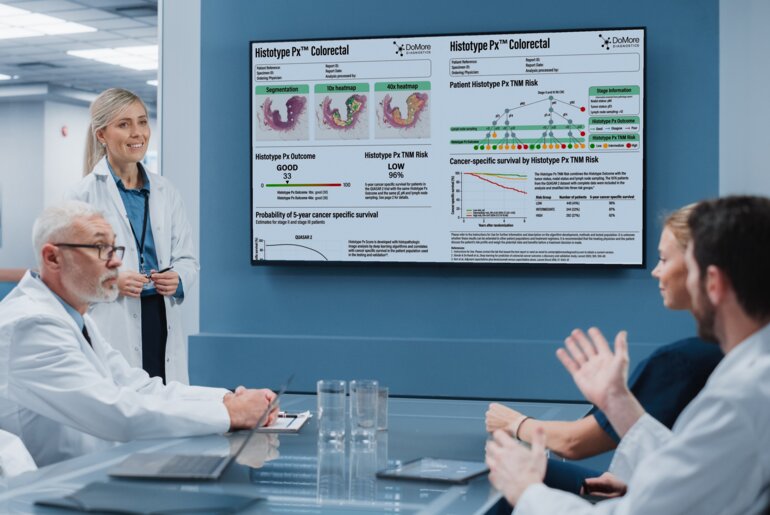
AI-driven digital pathology offers a promising solution to this challenge. With sufficient analysis and training on digitised routine H&E tumour slides using deep learning algorithms, advanced AI models can detect and quantify complex features beyond human perception and link these directly to patient outcomes (1). Studies show that AI-derived digital biomarkers can significantly enhance and outperform existing staging methods by stratifying patients into distinct outcome groups (2). These AI “digital biomarker” tools represent a new precision medicine modality, ensuring that patients receive the right care based on their individual risk profiles.
To ensure reliability, biomarkers must be robust and validated across diverse populations. Therefore, international clinical collaborations are essential to expand the clinical evidence for AI in digital pathology. Institutions in Japan (3), the USA (4,5), the Netherlands (6), the UK, France, Mexico, Denmark, and Norway are evaluating such AI-driven digital biomarkers. When combined with other modalities, such as circulating tumour DNA (ctDNA) testing, prognostic accuracy and guided therapy selection can be further improved (3,6).
AI digital biomarkers in pathology are cost-effective, have a short turnaround time and may provide higher precision than multi-gene assays. Moreover, digital biomarker AI analysis can be easily integrated into existing workflows which can further accelerate adoption in clinical practice.
As AI continues to redefine precision oncology, its application in digital pathology stands at a transformative moment. The technology is actively being developed for many cancer types, and the first AI digital biomarker test has entered NCCN clinical guidelines (7). While the journey toward full clinical integration is ongoing, the progress to date reflects the potential of AI to advance cancer care.
(1) Skrede OJ et al., Deep learning for prediction of colorectal cancer outcome: a discovery and validation study, Lancet. 2020 Feb 1;395(10221):350-360
(2) Kleppe A et al., A clinical decision support system optimising adjuvant chemotherapy for colorectal cancers by integrating deep learning and pathological staging markers: a development and validation study, Lancet Oncol. 2022 Sep;23(9):1221-1232
(3) Novel Clinical Decision Support System Optimizing Adjuvant Chemotherapy for Colorectal Cancer by Integrating Deep Learning and circulating tumor DNA molecular residual disease: GALAXY Histotyping
(4) Clinical validation of Histotype Px Colorectal in patients in a U.S. colon cancer cohort
(5) Histotype Px® Colorectal as an AI Biomarker to Guide Use of Adjuvant Therapy in Patients with Resected Colon Cancer
(6) Complementary value of digital pathology to ctDNA in risk stratification of stage III colon cancer patients receiving adjuvant chemotherapy
(7) NCCN Guidelines for prostate cancer
DoMore Diagnostics is a Norwegian-based precision oncology company leveraging AI to improve prognostic prediction in cancer care. Their lead product, Histotype Px® Colorectal, demonstrates how AI can be applied on digitized pathology H&E slides to risk-stratify patients and support personalized treatment decisions. https://www.domorediagnostics.com/products
Histotype Px is a registered trademark in the European Union. Trademark registration status may vary by country.