A benefit was observed in women with germline BRCA1/2 mutations when treated with the PARP inhibitor alone or in combination with apatinib
As reported in a randomised phase III trial presented at an ESMO Virtual Plenary on 9 May 2024, the oral poly (ADP-ribose) polymerase (PARP) inhibitor fuzuloparib showed to prolong progression-free survival (PFS) in women with HER2 negative metastatic breast cancer harboring a germline BRCA1/2 mutation when compared to standard chemotherapy. Positive results in terms of PFS were observed whether when the PARP inhibitor was administered alone or in combination with apatinib, a VEGFR2 inhibitor.
In the trial, 203 patients were randomised to receive standard chemotherapy (capecitabine or gemcitabine), fuzuloparib (150 mg, twice a day) or fuzuloparib plus apatinib (100 mg plus 500 mg respectively, twice a day). All participants were pre-treated with an anthracycline and/or a taxane, no more than two lines of previous chemotherapy regimens and at least one line of endocrine therapy for HR+ disease in the recurrent/metastatic setting.
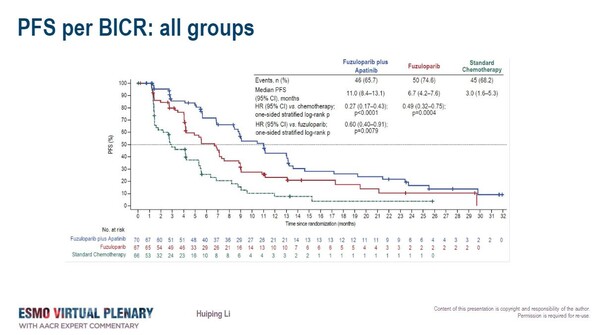
In both the monotherapy and the combination therapy arms, superiority in PFS with the PARP inhibitor compared to chemotherapy – the primary study endpoint – was reported. A greater PFS benefit was observed with fuzuloparib plus apatinib compared to chemotherapy (11.0 vs. 3.0 months; HR, 0.27; 95% CI, 0.17–0.43; P<0.0001) and compared to fuzulaparib alone (HR 0.60; 95%CI 0.40-0.91). Fuzuloparib alone was also better than chemotherapy (HR, 0.49; 95% CI, 0.32–0.75; P=0.0004).
“These results are interesting because the combination therapy yields better results than the PARP inhibitor alone”, says Dr Evandro de Azambuja from Jules Bordet Institute, Brussels, Belgium, commenting on the data presented. “A similar PFS benefit was observed in HR+ and triple negative breast cancer subgroups.” Also, a trend towards improved overall survival was observed with the PARP inhibitor compared to chemotherapy when administered alone (29.2 vs. 21.5 months; HR, 0.58; 95% CI, 0.33–1.02) or in combination with apatinib (31.5 vs. 21.5 months; HR, 0.61; 95% CI, 0.35–1.08).
Hematological and gastrointestinal toxicity were the most common toxicities observed in the study. Almost the totality of patients had at least one adverse event (AE) of any grade (98.6% in the fuzuloparib plus apatinib arm and 92.5% in the fuzuloparib arm), and half of them had grade ≥3 AEs (51.4% in the fuzuloparib plus apatinib arm and 49.3% in the fuzuloparib arm). “However, the addition of apatinib increased the rate of treatment discontinuation due to treatment-related AEs, and it was reported in 7.1% of patients treated with fuzuloparib plus apatinib compared to 0% and 3.4% of those in the fuzuloparib and in standard chemotherapy arm resepctively”, observed de Azambuja.
Fuzuloparib has been approved in China for the treatment of platinum-sensitive recurrent ovarian cancer, fallopian tube cancer or primary peritoneal cancer in patients with germline BRCA1/2 mutations who have undergone second-line or above chemotherapy. “Probably, this combination would be approved in Asia and help women with metastatic breast cancer with an alternative to chemotherapy. However, this indication should also be extended to patients harboring PALB2 mutations, for whom olaparib and talazoparib are also indicated”, concludes de Azambuja.