Safety and efficacy data of a PD-1/IL-2 bispecific antibody fusion protein were presented at an ESMO Virtual Plenary
Findings from a first-in-human phase I study investigating IBI363, a PD-1/IL-2 bispecific antibody fusion protein, provide proof-of-concept on its capacity to revert acquired resistance to immunotherapy in pre-treated patients and in some populations with a poor prognosis including patients with mucosal melanoma (VP4-2024).
In recent years, novel immunotherapy agents against several targets on cancer cells or the tumour microenvironment have been developed either as single agents or in combination with anti-PD-1/L1 inhibitors, proving their efficacy. “However, these efforts have been hampered by the high biological complexity of cancer-immune cell interactions within tumour ecosystems, and still there are few options to overcome intrinsic or acquired immune resistance in cold tumours or those progressing after standard PD-1/L1 blockade,” explains Dr Alberto Hernando-Calvo from Vall d´Hebron Barcelona Hospital Campus and the Vall d´Hebron Institute of Oncology (VHIO), Barcelona, Spain, during the ESMO Virtual Plenary on 13 June where the phase I data were presented.
IBI363 was designed to selectively deliver Interleukin 2 (IL-2) to activated tumour-specific T cells (TST) that co-express PD-1 and IL-2 receptor α (IL-2Rα), and is a potential candidate to overcome immune escape and resistance through reverting T-cell exhaustion (Nat Cancer. 2023 Sep;4(9):1309-1325).
In the study, a total of 22 patients with advanced or metastatic solid tumours intolerant or having progressed to standard therapies were enrolled in the dose escalation part, and 325 additional patients were treated in a dose expansion part exploring different dosing schedules (Q2W and Q3W). Colorectal cancer, non-small cell lung cancer (NSCLC) and melanoma were the most represented tumour types in the study. The majority of trial participants had received at least two prior lines of systemic therapies (81.8%), and two thirds of patients were previously treated with immunotherapy (67.7%).
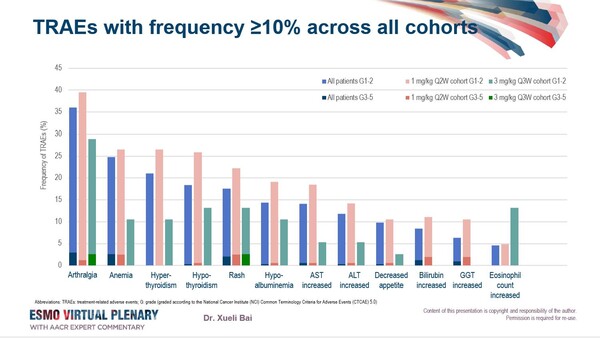
Overall, the treatment with the PD-1/IL-2 bispecific antibody fusion protein at doses ≥0.1 mg/kg was well tolerated. At data cutoff, most common treatment-related adverse events (TRAEs) included arthralgia (33.4%, with 3.1% ≥G3) and anaemia (22.7%, with 2.5% ≥G3) (Figure). TRAEs led to IBI363 discontinuation or death in 11 (3.2%) and 2 (0.6%) patients, respectively.
Although immature at data cutoff, an objective response rate (ORR) of 17.6% maintained across the immunotherapy pre-treated population was observed. “Also, ORR was 18.2% in the immunotherapy pre-treated population with wild-type NSCLC adenocarcinoma, and 35.1% in patients with squamous NSCLC in dose cohorts ≥0.3 mg/kg”, says Hernando-Calvo. “Interestingly, across patients with immunotherapy-naive mucosal melanoma treated with IBI363 at doses ≥0.3 mg/kg the ORR was 75%.”
Hernando-Calvo concludes. “Although additional work remains to be conducted to fully elucidate the subset of patients who may derive benefit from IBI363, the data presented by Dr Bai et al. support further investigation to pave the way for designing novel IL-2-based immunotherapy combinations in the anti-PD-1/L1 resistant setting.”