Findings suggest that analysis of multiple prostate diagnostic biopsy cores, in contrast to frequent practice, may help reduce tumour mischaracterisation and identify genetic alterations relevant to personalised treatment
A study presented at the Molecular Analysis for Precision Oncology (MAP) Congress 2022 reveals important information about the genomic heterogeneity of primary and metastatic samples from patients with de novo metastatic castration-sensitive prostate cancer (mCSPC) (Abstract 4MO). In total, 607 samples from synchronous primary foci, metastatic lesions and cell-free DNA from a unique clinical trial of 43 de novo mCSPC patients who underwent radical prostatectomy were subjected to targeted DNA sequencing using a bespoke prostate cancer-specific panel and/or whole-exome sequencing.
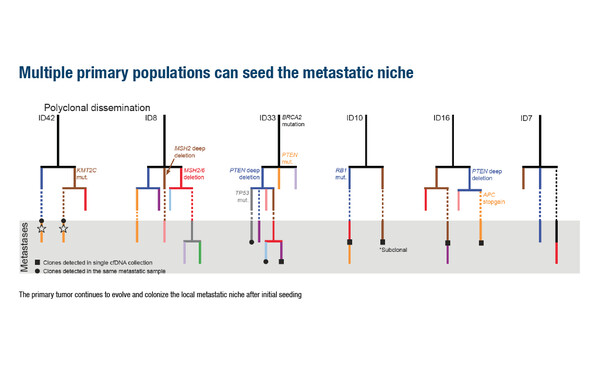
The researchers found that while samples appeared ideal for 2D pathologic evaluation, the 3D tumour fraction was low in 20% of patients across all foci. Importantly, intra-patient heterogeneity was common, in terms of mutations and copy number, with ploidy variation by region. Evolutionary branching was detected within the primary site and, of note, multiple primary populations were found to seed the metastatic niche.
Frequent discordance in clinically relevant genes was observed between select primary foci and synchronous metastases, plus highly variable per-sample tumour fraction. These findings suggest that false genotyping of the dominant disease may occur when relying on a single tissue focus. Mischaracterisation appeared to be reduced by sequencing DNA from multiple samples, which improved the detection rate of patients’ true genotype.
Commenting on the study and its results, Prof. Elena Castro from the Hospital Universitario 12 de Octubre, Madrid, Spain, notes: “Prostatectomy is not the standard of care in the metastatic hormone-sensitive setting and the only tissue available is usually from the prostatic biopsy at diagnosis. In the context of a clinical trial, the researchers were able to analyse and compare samples from multiple foci within primary tumours, from metastatic sites and from circulating free DNA, which is unique.”
Discussing the main conclusion, she says, “mCSPC has been understudied so far. It appears that the genomic landscape of mCSPC is more similar to that of castration-resistant prostate cancer than we had thought. Importantly, these analyses provide the first demonstration of the polyclonality of metastases in mCSPC, which has previously been shown in castration resistant disease. This study demonstrates that if we analyse only a single sample, as we usually do, it may not be representative of the main drivers of the tumour – multiple samples are needed to identify the genetic alterations of relevance. Although we do not have targeted therapies for mCSPC at the moment, various trials are ongoing. When those results are available, accurate knowledge of a tumour’s main genomic alterations will be very relevant.”
Abstract presented:
Murtha AJ, et al. Multi-focal genomic dissection of synchronous primary and metastatic tissue from de novo metastatic prostate cancer. MAP 2022, Abstract 4MO
Mini Oral Session 16.10.22, h. 15:40 – 16:40, Auditorium
Watch the session on demand on the Congress virtual platform