However, a higher objective response rate with atezolizumab was reported in the IMpassion132 trial suggesting that at least some patients could benefit from it
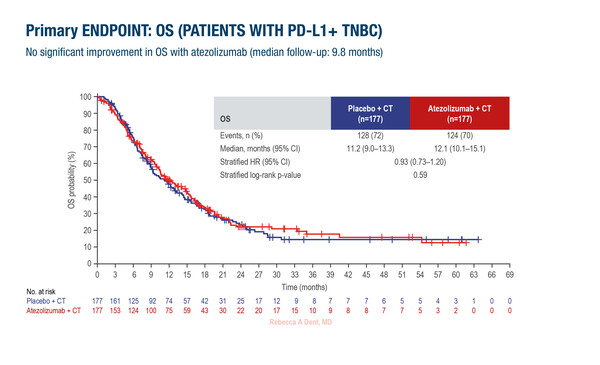
At ESMO Breast Cancer 2024 (Berlin, 15–17 May), long-awaited results from the IMpassion132 trial revealed that adding atezolizumab to chemotherapy did not prolong survival versus placebo in PD-L1-positive patients with advanced triple-negative breast cancer (TNBC) relapsing within 12 months after anthracycline-/taxane-containing neoadjuvant chemotherapy or primary surgery (Abstract 180O; Ann Oncol. 2024; Article in press).
A total of 354 patients were involved in the trial. Median overall survival was 12.1 months with atezolizumab plus chemotherapy and 11.2 months with placebo plus chemotherapy (stratified hazard ratio 0.93; 95% confidence interval 0.73–1.20; p=0.59). The median follow-up was 9.8 months. The median progression-free survival was also similar between treatment arms, at around 4 months, but objective response rates (ORRs) were 40% with atezolizumab and 28% with placebo. Adverse events, which were predominantly haematological, were broadly comparable between arms.
“The lack of atezolizumab benefit on survival indicates a continued poor prognosis for patients with early relapsing advanced TNBC,” says Dr Carmen Criscitiello from the European Institute of Oncology, Milan, Italy. “However, the higher ORR achieved with atezolizumab suggests that at least some patients could benefit from it, and this highlights the importance of individualised treatment approaches.”
Criscitiello thinks that the identification of biomarkers that predict treatment response is needed for designing more effective clinical trials and personalised treatment plans. “Developing reliable, accessible and cost-effective diagnostic tests for potential biomarker assessment is difficult – we have not yet achieved this and translating theory into clinical practice is considerably more complex,” she cautions. Likewise, the study presenters suggest that a biology-based definition of intrinsic resistance to checkpoint inhibitors is crucial and Criscitiello agrees, but notes that this will be challenging given the varied genetic, molecular and immunological profiles of such a heterogeneous disease.
“Pending the development of targeted therapies based on the molecular characteristics of TNBC, new hope for patients may also be offered by combination regimens including antibody–drug conjugates and next-generation immune checkpoint inhibitors, with more clinical trials urgently required,” concludes Criscitiello.
Abstract discussed
Dent RA, et al. IMpassion132 double-blind randomised phase 3 trial of chemotherapy (CT) ± atezolizumab (atezo) for early-relapsing unresectable locally advanced or metastatic triple-negative breast cancer (aTNBC). ESMO Breast Cancer 2024, Abstract 180O
Proffered Paper Session 1, 15.05.2024, h. 16:45 – 17:55, Berlin Hall