Findings from the PACIFIC-6 trial echo the benefits seen with durvalumab after concomitant chemoradiotherapy, representing a potential alternative for vulnerable and older patients
Results from the phase II PACIFIC-6 trial presented at the European Lung Cancer Congress 2022 show that the safety profile of durvalumab treatment who had not progressed after sequential chemoradiotherapy (sCRT) was similar to that observed after concurrent chemoradiotherapy (cCRT) (Abstract 108MO).
Durvalumab is recommended by ESMO Guidelines (eUpdate) for the treatment of unresectable stage III non-small-cell lung cancer (NSCLC) that has not progressed following cCRT based on results of the phase III PACIFIC trial (J Clin Oncol. May 20, 2021. 39, no. 15_suppl 8511-8511). However, not all patients are eligible for cCRT – particularly older or more frail individuals who may not tolerate concurrent therapy based on their functional status – and sCRT is an accepted alternative.
In the PACIFIC-6 trial, across 117 patients with stage III NSCLC who received durvalumab after sCRT, the primary endpoint of the incidence of grade 3/4 possibly related adverse event (PRAEs) within 6 months of starting durvalumab was experienced by 5 (4.3%) patients. All grade 3-4 PRAEs that were reported in the trial occurred within 6 months of treatment initiation and two of these events were grade 3-4 pneumonitis. Over the median total treatment duration of 32.0 weeks, 76.9% of patients had any-grade PRAEs and 18.8% had any grade 3-4 AEs. AEs and PRAEs resulted in treatment discontinuation in 21.4% and 16.2% of patients, respectively, with pneumonitis reported as the most common AE leading to treatment discontinuation (10.3%). There were two fatal AEs, one of which was a PRAE (pneumonitis). Within the study population, the median age was 68.0 years, 98.3% had past/present medical conditions (mainly vascular [59.0%] or respiratory [53.8%] disorders) and 3 patients were included with a World Health Organization/Eastern Cooperative Oncology Group performance status (WHO/ECOG PS) of 2.
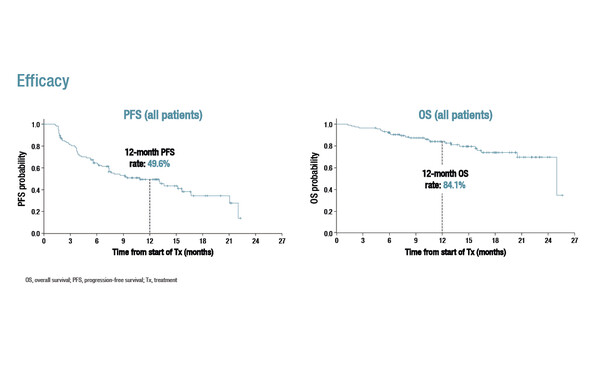
Regarding efficacy, median progression-free survival (PFS) was 10.9 months (95% confidence intervals [CI] 7.3–15.6) and the 12-month PFS rate was 49.6%. Median overall survival (OS) was 25.0 months (95% CI 25.0–not calculable), with a 12-month OS rate of 84.1%. There was a confirmed objective response rate of 17.1%.
Commenting on the study and its findings, Prof. Jarushka Naidoo from Beaumont Hospital, RCSI University of Health Sciences, Dublin, Ireland and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD, USA, says, “We know that stage III NSCLC is a heterogenous disease, and that not all patients are suitable candidates for cCRT. PACIFIC-6 addressed a clinically relevant question and the data are reassuring given that the majority of patients were ≥65 years old and patients with WHO/ECOG PS 2 were included, albeit a small number. Specifically, the incidence of all toxicities including high-grade pneumonitis was similar to that in PACIFIC, and early efficacy results are encouraging. This adds to the exciting data on the growing use of immunotherapy to treat early-stage cancers.”
Garassino MC, et al. Safety and efficacy outcomes with durvalumab after sequential chemoradiotherapy (sCRT) in Stage III, unresectable NSCLC (PACIFIC-6). European Lung Cancer Congress 2022, Abstract 108MO
Mini Oral Session 1, 31.03.2022, h. 17:00 – 18:00, South Hall 2