Health-related quality of life data from the POSEIDON study support previous efficacy findings for the benefits of a two-agent immunotherapy regimen plus chemotherapy over chemotherapy alone
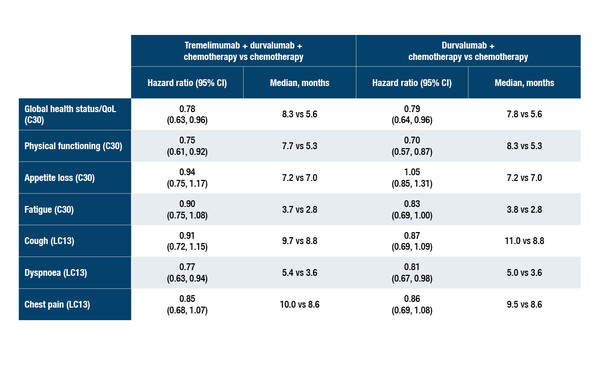
In data presented at the European Lung Cancer Congress 2022, the risk of time to deterioration (TTD) of global health status/quality of life (QoL), assessed with EORTC QLQ-C30/LC13, was reduced (hazard ratio 0.78) with the triple regimen of tremelimumab plus durvalumab plus chemotherapy compared with chemotherapy alone in patients with EGFR/ALK wild-type metastatic non-small-cell lung cancer (NSCLC) (Abstract 5MO). TTD was prolonged for most symptoms/domains – including physical functioning and dyspnoea – with the triple regimen compared with chemotherapy. These patient-reported outcomes (PROs) data follow the reporting of the POSEIDON efficacy data in September 2021.
The improvement in TTD scores compared with chemotherapy alone was evident for both tremelimumab plus durvalumab plus chemotherapy and durvalumab plus chemotherapy across most symptoms/domains investigated. There was also an increase in the rates of improvement seen with triple- and dual-combination therapy compared with chemotherapy.
The POSEIDON study randomised 1,013 patients 1:1:1 to receive first-line treatment with either: tremelimumab plus durvalumab plus platinum-based chemotherapy, then durvalumab every 4 weeks until progression; durvalumab plus chemotherapy, followed by durvalumab until progression; or chemotherapy. TTD was analysed using a stratified log-rank test with a Cox proportional-hazards model. In this current analysis, compliance with PRO reporting was ≥60% for up to 88 weeks with the triple combination, 64 weeks with the dual combination and 24 weeks with chemotherapy. Baseline values were similar across treatment arms.
Data presented in 2021 demonstrated that the triple immunotherapy-based combination significantly improved overall survival (OS) and progression-free survival compared with chemotherapy alone (J Thorac Oncol. 2021;16(C10 Suppl):S844). A trend for OS improvement with the dual combination over chemotherapy alone did not reach statistical significance. The incidence of treatment-related adverse events (TRAEs), and discontinuations due to TRAEs, were numerically higher in the triple-combination arm compared with the chemotherapy arm.
Abstract discussed:
Garon EB, et al. Patient-reported outcomes (PROs) with 1L durvalumab (D), with or without tremelimumb (T), plus chemotherapy (CT) in metastatic (m) NSCLC: Results from POSEIDON. European Lung Cancer Congress 2022, Abstract 5MO
Mini Oral Session 2, 30.03.2022, h. 14:15 – 15:15, Club A