Results from the subgroup analysis of the IMpower010 study also report some patterns of relapse that are in contrast with previously presented findings
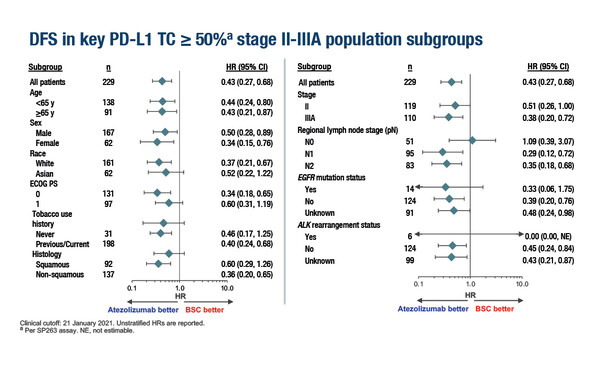
A new exploratory analysis of the IMpower010 study, presented at the European Lung Cancer Congress 2022, suggests that atezolizumab may prolong disease-free survival (DFS) across subgroups of patients with PD-L1 expression ≥50% and patterns of relapse hint at a disease-modifying effect of immunotherapy. The interim unstratified DFS analysis reports that the addition of atezolizumab to adjuvant chemotherapy improves the risk of DFS by 40–71% compared with best supportive care (BSC) across subgroups of age (<65 years and ≥65 years), sex, race (White and Asian), Eastern Cooperative Oncology Group performance status (0 or 1), smoking status, histology (squamous and non-squamous), stage (II and IIIA), regional lymph nodes (N1, N2) and detection of EGFR mutations among patients with stage II–IIIA non-small-cell lung cancer (NSCLC) and PD-L1 expression ≥50% (Abstract 80O). There was no apparent benefit of atezolizumab over BSC for patients with no regional lymph node involvement (N0) (hazard ratio [HR] 1.09).
Fifty patients (44%) receiving BSC and 25 (22%) receiving atezolizumab relapsed. Distant-only relapse was more common with BSC (21 patients, 18%) than with atezolizumab (6 patients, 5%). A similar finding was made for CNS-only relapse (7 [6%] versus 1 [1%]). Locoregional-only relapse was seen in 17 patients (15%) receiving BSC and 15 (13%) receiving atezolizumab. Systemic therapy following relapse was received by 19 patients (17%) receiving atezolizumab and 30 patients (26%) receiving BSC. In the atezolizumab arm, subsequent therapy comprised chemotherapy for 15 patients (13%) and immunotherapy for 4 patients (3%). Corresponding figures for the BSC arm were 16 (14%) and 19 patients (17%).
Safety was consistent with that of the overall study population and the known safety profile of atezolizumab.
The primary analysis of the phase III IMpower010 study had previously reported that the reduction in the risk of recurrence achieved by adding atezolizumab to adjuvant chemotherapy for stage II–IIIA resected NSCLC was greatest in the subgroup of patients with PD-L1 expression ≥50% (unstratified HR 0.43) (Lancet. 2021;398:1344–1357). Within this subgroup, baseline characteristics were generally balanced for sex, stage and histology between the treatment arms.
“The results of the subgroup analysis are promising, and suggest little heterogeneity in the efficacy of atezolizumab across high PD-L1 expressers. However, given the very small patient numbers in some of the subgroups, the results have to be treated with caution,” says Prof. Noemi Reguart from the Hospital Clinic Barcelona, Spain.
“What is perhaps more interesting are the patterns of relapse reported in the analysis,” continues Reguart. “Distant relapse and CNS relapse were common with BSC, whereas most relapses in the atezolizumab arm were locoregional-only. These data contrast with the post-hoc analysis presented at ESMO 2021, in which patterns of relapse – locoregional, distant, CNS or second primary lung – were similar in stage II–IIIA PD-L1-positive disease, the all-stage II–IIIA population, and the intention-to-treat stage IB–IIIA population (Ann Oncol. 2021;32(Suppl5):S1283–S1346). Although we are talking about small sample sizes, the results are intriguing. It might be possible that we are witnessing an increased systemic- and CNS-protective effect in these groups of high PD-L1 expressors, in whom atezolizumab could be changing the course of biological disease.” Reguart points out that this effect has also been reported in patients with resected EGFR-mutated NSCLC receiving adjuvant osimertinib (N Engl J Med. 2020;383:1711–1723). If true, it could have important implications for therapy, given that locoregional relapse is more amenable to radical treatment and, as such, is associated with a greater chance of cure. “It will be interesting to see if the trend in the relapse pattern with atezolizumab in patients with PD-L1 expression ≥50% is confirmed with a higher number of events and a longer follow-up,” she concludes.
Abstract presented:
Felip E, et al. Atezolizumab (atezo) vs best supportive care (BSC) in stage II-IIIA NSCLC with high PD-L1 expression: sub-analysis from the pivotal phase III IMpower010 study. European Lung Cancer Congress 2022, Abstract 80O
Proffered Paper Session, 31.03.2022, h. 15:15 – 16:35, Congress Hall