Early-phase findings report manageable toxicity when combining the PARP inhibitor with the anti-angiogenesis inhibitor in two cohorts of patients with ovarian and castration-resistant prostate tumours
Results from an arm of the investigator-initiated TalaCom study provide early evidence in favour of combining the PARP1/2 inhibitor talazoparib with the VEGFR inhibitor axitinib in patients with BRCA1/2 wildtype high-grade serous ovarian cancer (HGSOC) or molecularly unselected metastatic castration-resistant prostate cancer (mCRPC). Findings were presented at the ESMO Targeted Anticancer Therapies Congress 2025 (Paris, 3–5 March) (Abstract 28O).
The rationale behind testing the combination relates to the suggestion that angiogenic inhibition may potentially increase sensitivity to PARP inhibitors (Neoplasia. 2014;16:343–353), which may help to expand the use of talazoparib beyond approved indications in BRCA-mutated breast cancer and homologous recombination repair-deficient CRPC.
The phase Ib TalaCom trial aims to determine the best dose, possible benefits and/or side effects of talazoparib when given in combination with palbociclib, axitinib or crizotinib for the treatment of patients with locally advanced or metastatic solid tumours (NCT04693468). In arm B, 28 patients with advanced cancer with or without DNA damage response mutations initially received talazoparib 1 mg once daily and axitinib 5 mg twice daily during the dose-escalation period. The axitinib dose was adjusted based on individual patient tolerability every 2 weeks up to 10 mg twice daily or was lowered if needed. The talazoparib dose was reduced to 0.75 mg, if appropriate, after axitinib dose alterations. The most common grade ≥3 treatment-related adverse events with talazoparib plus axitinib were decreased platelet count (32%), hypertension (25%), decreased neutrophil count (18%) and anaemia (18%).
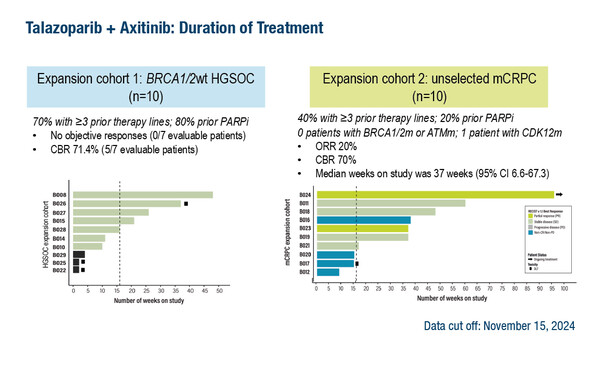
In dose-expansion cohorts, an objective response rate of 20% and a clinical benefit rate of 70% was observed among 10 efficacy-evaluable patients with molecularly unselected mCRPC. Among 10 patients with BRCA1/2 wildtype HGSOC, there were no objective responses, but 71.4% of patients derived clinical benefit. Patients in these cohorts had been heavily pretreated, many with prior PARP inhibitor therapy. They all initiated treatment on talazoparib 1 mg once daily and axitinib 5 mg twice daily. One patient remained on study at data cut-off. Biomarker and pharmacokinetic analyses are underway.
Programme details
Ngoi NY, et al. TalaCom: Phase Ib investigator-initiated trial combining talazoparib and axitinib in patients with DNA damage response mutated cancers or BRCA1/2 wildtype ovarian cancer incorporating prospective intrapatient dose titration. ESMO Targeted Anticancer Therapies Congress 2025, Abstract 28O
Oral Abstract Session 1, 03.03.2025, h. 15:45 – 17:15, Scene AB