Positive in vitro and in vivo antitumour effects in breast and lung cancer models and in mismatch repair-driven cancer models
New research suggests that the future is bright for the use of CDK 4/6 inhibitors based on preclinical results with novel agents and in new treatment settings, presented today at the ESMO Congress 2021.
In the first preclinical study, the antiproliferative activity of GLR2007, a novel CDK4/6 inhibitor, was shown in vitro against a panel of breast and lung cancer cell lines. Greater potency with GLR2007 was observed over another CDK4/6 inhibitor, abemaciclib, with lower half maximal inhibitory concentration (IC50) values with GLR2007 in 5 out of 7 human and murine breast cancer cell lines, and 20 out of 21 human lung cancer cell lines (Abstract 4MO). In vivo, significant tumour growth inhibition against breast and lung cancer xenograft models was recorded with GLR2007 but not abemaciclib. Commenting on these data, Dr Geoffrey Shapiro of the Dana-Farber Cancer Institute, Boston, MA, USA says, “This extensive preclinical work suggests that GLR2007 can reduce the proliferation of breast cancer and lung cancer cell lines compared with another CDK4/6 inhibitor at lower concentrations, and it had greater antitumour activity in vivo. While these data are very encouraging, it is still to be determined whether these early findings with GLR2007 will translate into superior efficacy over other CDK4/6 inhibitors in a clinical setting.”
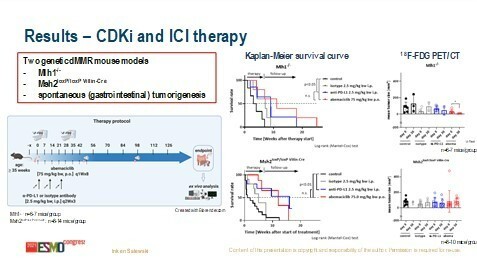
Shapiro continues, “What is particularly exciting about GLR2007 is its greater penetration of the blood–brain barrier than the other CDK4/6 inhibitors.” Previous preclinical analyses in rat models have shown that GLR2007 has good distribution in the brain and superior activity against orthotopic glioblastoma models than abemaciclib (J Clin Oncol. 2021;39[15_Suppl]:e14023). “Taking these preclinical data together, GLR2007 has the potential to be added to the CDK4/6 armamentarium,” continues Shapiro, “The good biochemical, preclinical drug distribution, in vivo and in vitro activity justify the clinical evaluation of this drug in both glioblastoma and other solid tumours, and we look forward to these data.” Results from a preclinical in vivo study suggests that CDK4/6 blockade is as effective as immune checkpoint inhibition (Abstract 5MO). The study analysed an anti-PD-L1 antibody versus abemaciclib in two immunocompetent murine models of mismatch repair (MMR)-driven carcinogenesis. Both treatments significantly prolonged overall survival in both models harbouring MLH1 and MSH2 mutations versus controls, and one complete remission was observed following abemaciclib administration, whereas the best response with anti-PD-L1 antibody administration was stable disease. CDK4/6 inhibition with abemaciclib induced changes in the tumour microenvironment, including increased numbers of T-cells and PD-L1 upregulation. According to Shapiro, these data are incredibly compelling. “We know that MMR-driven cancers can have a high mutational burden or high neoantigen load and are very responsive to PD-L1 blockade. These novel data show that the MMR-deficient murine models are also very sensitive to CDK4/6 inhibition, suggesting that CDK4/6 inhibitors may have a role in tumours with this type of alteration.”
We need to address the issue of resistance to CDK4/6 inhibitors which, like most targeted therapies, is universal.
The field of CDK4/6 research is very exciting and moving forward rapidly and the story with combined CDK4/6 inhibitor and immune checkpoint inhibitor is not over. Recent studies also showed that CDK4/6 inhibitors can stimulate immune memory (Cancer Discov 2021 May 3:candisc.1540.2020) (Cancer Discov 2021 May 14:candisc.1554.2020). “Proving that CDK4/6 inhibitors have long-lasting effects on the immune microenvironment would be very positive. Previous combinations of a CDK4/6 inhibitor with immune checkpoint blockade have stalled because of toxicity issues. However, this could potentially be addressed if CDK4/6 inhibitors affect immune memory, as changes in the tumour microenvironment could be induced without the need for continuous treatment with the CDK4/6 inhibitor.”
Future research with combinations of CDK4/6 inhibitors and inhibitors of different signal transduction pathways will be key, according to Shapiro. “There may be novel schedules that need to be evaluated. One approach would be to optimise these in MMR-driven cancer models where the known clinical effects of immune checkpoint blockade in these tumours could be augmented by administration of a CDK4/6 inhibitor. Importantly, we need to address the issue of resistance to CDK4/6 inhibitors which, like most targeted therapies, is universal. What is emerging in the literature is the heterogeneity of resistance via activation of multiple signal transduction pathways that can overcome the cell cycle arrest that CDK4/6 inhibitors afford.”
Yin L et al. Preclinical evaluation of novel CDK4/6 inhibitor GLR2007 in breast and lung cancer models. ESMO Congress 2021, Abstract 4MO
Mini oral session – Basic science, 19.9.2021, h. 17:30 – 17:35, Channel 4
Salewski I et al. CDK4/6 blockade is as effective as immune-checkpoint inhibition in tumor growth control of Mlh1-/- and Msh2loxP/loxP villin-Cre mice. ESMO Congress 2021, Abstract 5MO
Mini oral session – Basic science, 19.9.2021, h. 17:35 – 17:40, Channel 4