CPL304110 shows acceptable toxicity and early signs of activity in heavily pre-treated patients with advanced solid malignancies
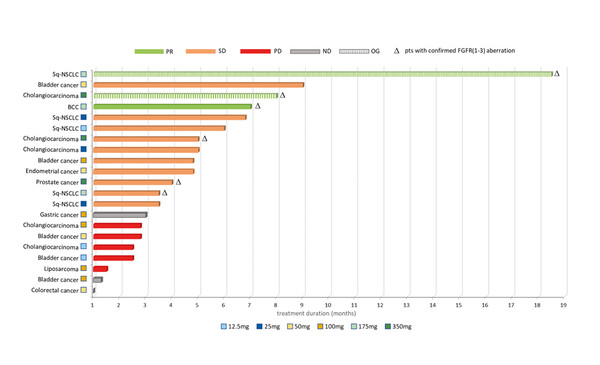
According to results from a phase IA trial presented at the ESMO Targeted Anticancer Therapies Congress 2023 (Paris, 6–8 March), treatment with a new tyrosine kinase inhibitor of fibroblast growth factor receptor (FGFR) 1–3, CPL304110, led to a partial response in three out of 21 patients receiving the oral therapy (Abstract 46O). Cross-tumour benefits were observed in squamous cell lung cancer, basal cell carcinoma and cholangiocarcinoma.
This resulted in a response rate of 14.3% among all patients (those with aberrations in tumour FGFR and those not screened for tumour FGFR aberrations), which rose to 50.0% among patients with tumour FGFR aberrations. Stable disease was observed in 10 patients.
CPL304110 was administered every 28 days at escalating doses of 12.5 mg to 100 mg once daily in patients not screened for tumour FGFR aberrations and at a dose of 175 mg once or twice daily in patients with documented tumour FGFR aberrations. In the overall population, the most common treatment-related adverse events (TRAEs) included ocular toxicity (23.8%), anaemia (19.0%), dry eyes and dry mouth (both 14.3%). At the time of reporting, there were two incidences of grade ≥3 TRAE (oral cavity fungal infection and alkaline phosphatase increase). Preliminary results suggest a low potential for CPL304110 accumulation and linear pharmacokinetics. The recommended phase II dose will be selected following completion of the phase IB part of the trial.
“In addition to the promising efficacy findings from this trial, the adverse event profile of CPL304110 appears particularly encouraging,” says Prof. Christian Rolfo from the Tisch Cancer Institute, New York, NY, USA. “While FGFR inhibitors have transformed the care of patients with tumours harbouring FGFR aberrations, the widespread involvement of FGFR in physiological pathways meant that the early-generation, relatively non-specific, inhibitors were limited by sometimes severe toxicity. So, it is reassuring that the safety profile of CPL304110 was in line with that of the new, more specific inhibitors and that only two patients in this phase I trial developed a TRAE of at least grade 3,” he says. “Clearly, we need more data before we can begin to speculate on the value of CPL304110 in the clinic, but it is worth noting that it will be entering the field behind other enhanced-specificity agents, such as the FGFR 1–4 inhibitor, futibatinib. So, it will be important to gain an understanding of how the different FGFR inhibitors can best be used, in terms of sequencing and in combination with other treatments,” observes Rolfo. He adds, “Exploratory analyses on FGFR aberrations will be critical to further define the subgroup of patients that derives the greatest benefit from this agent.”
Looking ahead, the success of FGFR inhibitors in clinical practice relies on the accurate identification of tumour FGFR aberrations and Rolfo thinks that improved technologies, such as whole-genome sequencing and circulating tumour (ct)DNA analysis, will be critical to achieving this. “The inevitable development of resistance is another major challenge, and the use of novel strategies, such as the concomitant use of proteolysis inhibitors, may help to overcome this,” he concludes.
Abstract discussed:
Lugowska I, et al. Preliminary results from a phase IA trial of selective FGFR1-3 inhibitor CPL304110 in patients with FGFR-deregulated advanced solid malignancies. ESMO Targeted Anticancer Therapies Congress 2023, Abstract 46O
Proffered Paper Session, 07.03.2023, h. 17:05 – 18:20, Amphitheatre Bordeaux