Lurbinectedin and irinotecan demonstrate a synergic clinical effect in patients with neuroendocrine carcinomas of gastroenteropatic primary site after chemotherapy failure
As presented at the ESMO Sarcoma and Rare Cancers Congress 2024 (Lugano, Switzerland, 14–16 March), findings from an expansion cohort of the phase I/II, multicenter, open-label PM1183-A-014-15 study reported that the combination of irinotecan and lurbinectedin is active in patients with pre-treated Grade 3 (G3) gastroenteropancreatic neuroendocrine carcinomas (GEP-NECs) (Abstract 27O).
Lurbinectedin is a novel anti-cancer agent that inhibits trans-activated transcription and modulation of the tumor microenvironment. In 2020, it was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with progressing metastatic small-cell lung cancer or after platinum-based chemotherapy based on the results of a phase II study (Lancet Oncol. 2020 May;21(5):645-654). A synergic effect of lurbinectidin and irinotecan, which acts at a different level of transcription, was previously observed in pancreatic cancer (Curr Oncol. 2023 Nov; 30(11): 9611–9626).
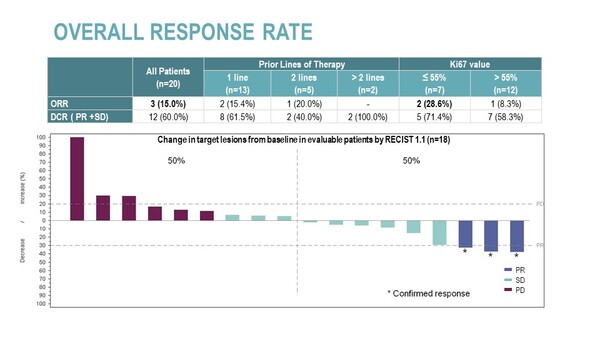
At phase II expansion stage of the study, 20 patients with G3 (Ki67>20%) poorly differentiated NECs of GEP or unknown primary site after progression to platinum-based therapy were treated with lurbinectedin 2.0 mg/m2 plus irinotecan 75 mg/m2 with primary G-CSF prophylaxis.
Confirmed partial response (PR) was reported in 3 pts (ORR=15%) and disease stabilization (SD) in 9 pts (DCR=60%). A longer progression-free survival (PFS) than that observed with prior therapy was reported in 25% of patients, with median PFS of 3.1 months (95%CI, 1.4-5.6). Median overall survival (OS) was 7.2 months (95% CI, 2.9-NR), OS at 6 months was 55% (95% CI, 33.2%-76.8%) and OS at 12 months was 45.0% (95% CI, 23.2%-66.8%).
Regarding the safety profile, the combination therapy was well tolerated, and the pharmacokinetic analysis showed no evidence of drug-drug interaction.
Based on the presented findings, lurbinectedin in combination with irinotecan could represent a new promising regimen for treating patients with GEP-NECs who have failed previous platinum therapy, but further evidence from research is needed.