Uninterrupted imatinib is essential to optimise outcomes in metastatic and high-risk resected GIST
ESMO-EURACAN-GENTURIS guidelines recommended that imatinib is given indefinitely for metastatic gastrointestinal stromal tumour (GIST) (Ann Oncol. 2022;33:20–33); however, the feasibility of treatment interruption with reintroduction at progression was unknown. As presented at the ESMO Sarcoma and Rare Cancers Congress 2024 (Lugano, Switzerland, 14–16 March), imatinib interruption in non-progressing patients with advanced GIST was associated with shorter survival and faster emergence of resistance in the long term (Abstract 52O). Patients in the BFR14 study with complete or partial response, or stable disease at three time periods (1 year [n=57], 3 years [n=49] and 5 years [n=27]) were randomised to imatinib discontinuation and restart at progression (STOP arm) or to imatinib continuation until progression (CONT arm). The primary endpoint of progression-free survival was significantly improved in the CONT versus the STOP arm for patients randomised at 1 year (p<0.001), 3 years (p<0.001) and 5 years of imatinib (p=0.002). Time to imatinib resistance was significantly longer in the CONT versus STOP arm in patients randomised at 3 years (p=0.009) and 5 years (p=0.04). Overall survival (OS) after randomisation at 3 years was significantly longer in the CONT versus STOP arm (p=0.01), with no significant difference seen with randomisation at 1 or 5 years.
Prof. Bernd Kasper from Mannheim University Medical Center, Germany, comments: “The main message from the cleverly designed BFR14 study is clear: Do not stop imatinib in advanced GIST, even if patients have stable disease or a partial response, because interruption leads to worse outcomes. Imatinib should be given as a continuous therapy.”
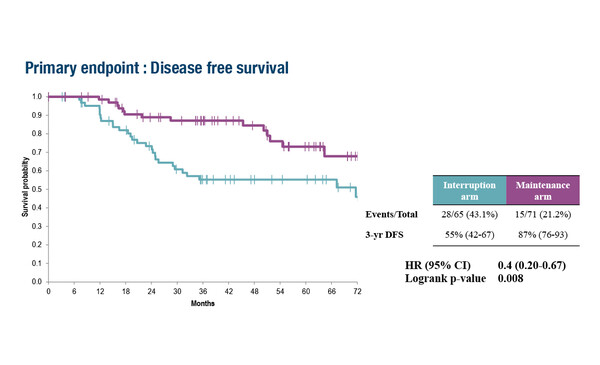
In another study, compelling evidence was provided for extending adjuvant imatinib to 6 years in completely resected, high-risk, KIT-positive GIST (Abstract 55O). In the phase III IMADGIST trial, all patients initially received imatinib for 3 years, then patients were randomised to either maintenance treatment for a further 3 years (n=71) or imatinib interruption with reintroduction at progression (n=65). The primary endpoint of disease-free survival (DFS) was significantly prolonged in patients on maintained versus discontinued treatment (3-year DFS of 87% and 55%, respectively; hazard ratio 0.40; 95% confidence interval 0.20–0.67; p=0.008). The risk of developing resistance to imatinib did not increase with the prolonged 6-year regimen (7 events) compared with interruption (8 events). OS data are immature. The incidence of muscle spasms was greater in the maintenance arm than the interruption arm (51% versus 22%; respectively), but events were rarely grade ≥3 (1% versus 0%, respectively).
Kasper says: “ESMO-EURACAN-GENTURIS guidelines recommend that imatinib is given for 3 years for adjuvant treatment of high-risk, completely resected localised disease (Ann Oncol. 2022;33:20–33), based on results from the SSGXVIII/AIO trial, which showed that 3 years of adjuvant imatinib therapy resulted in longer survival than 1 year of therapy (J Clin Oncol. 2016;34:244–250). Results from the IMADGIST trial provide high-level evidence that extending the duration of adjuvant imatinib treatment beyond 3 years to 6 years may be beneficial. These findings are likely to prompt reconsideration of the standard duration of imatinib use in the adjuvant setting in patients with KIT-positive high-risk resected GIST. The ongoing SSGXXII study (NCT02413736), which is comparing 3 years of adjuvant imatinib with 5 years, will also provide important information about imatinib in this context, but the results are expected for 2026.”
Abstracts discussed:
Blay J-Y, et al. Resistance to imatinib induced by treatment interruption in advanced GIST: long term outcome of the randomized BFR14 study. ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 52O
Proffered Paper Session 1, 14.03.2024, h. 10:30 – 12:00, Hall B1
Le Cesne A, et al. IMADGIST: A randomized study of 6 vs 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 55O
Proffered Paper Session 2, 14.03.2024, h. 16:15 – 17:45, Hall A