Results underline the need to further characterise dysbiosis, and to develop diagnostic and actionable tools for mainstream integration
The impact of gut microbiota on immunotherapy response and resistance has been established, but studies presented at the ESMO Immuno-Oncology Congress 2024 (Geneva, 11–13 December) highlight important associations between microbiota and immunotherapy-related toxicities.
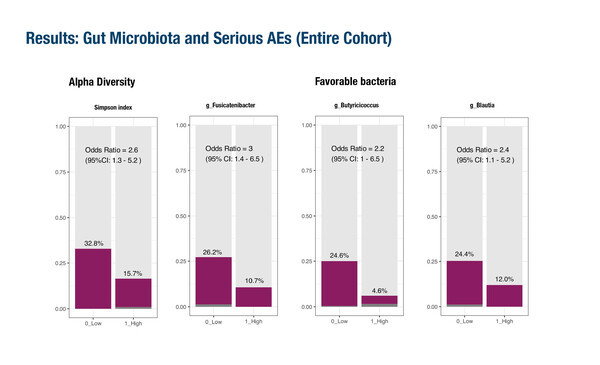
The first study, in 270 patients receiving pembrolizumab or nivolumab and ipilimumab plus platinum-based chemotherapy for non-small cell lung cancer (NSCLC), revealed that specific bacterial genera were associated not only with overall survival (OS) but also with serious adverse events (SAEs) (Abstract 1O). Low microbial diversity and distinct microbiota signatures, particularly lower abundance of butyrate-producing bacteria, such as Fusicatenibacter and Butyricicoccus, were associated with a higher incidence of SAEs.
In addition, the impact of the microbiota composition on SAEs and OS appeared to differ depending on the immunotherapy regimen used. For example, high levels of favourable bacteria appeared to be associated to superior OS with nivolumab and ipilimumab compared with pembrolizumab, while high levels of unfavourable bacteria resulted in shorter survival with nivolumab and ipilimumab than with pembrolizumab. The study was an analysis of the phase III Japanese NIPPON trial, which terminated patient accrual prematurely due to a high proportion of treatment-related deaths in the nivolumab and ipilimumab arm (Lancet Respir Med. 2024;12:877–887).
A second study, which used an animal model of melanoma, demonstrated an association between abnormal gut microbiota and PD-1 inhibitor-induced myocarditis via suppression of intracardiac regulatory T-cells (Abstract 46P). Removal of gut microbiota prior to PD-1 administration attenuated myocarditis, but enrichment of gut microbes after PD-1 inhibition showed a strong correlation with factors involved in myocarditis development.
Commenting on these findings, Prof. Laurence Zitvogel from the Gustave Roussy Cancer Centre, Villejuif, France, says, “These presentations both highlight an important and much less-studied relationship between microbiota and immunotherapy-related toxicities. The implications are that if unfavourable microbiota can be identified, bacteria-specific phages or antibodies could be given before immunotherapy to reduce the risk of SAEs.” Detecting unfavourable species has implications for faecal microbiota transplantation. “We need to ensure that unfavourable species are not included in faecal microbiota transplants. In the future, we could contemplate using phages, vaccines or monoclonal antibodies against identified pathogens to eliminate them from faecal microbial product before fecal transplantation,” highlights Zitvogel. Given the poor outcomes of patients with PD-1 inhibitor-induced myocarditis, she thinks a greater understanding of the mechanism is important: “We know that peptide mimics from certain commensal gut bacteria can promote inflammatory cardiomyopathy (Science. 2019;366:881–886) but it is also possible that bacterial metabolites, such as trimethylamine-N-oxide (TMAO), are involved (Int J Mol Sci. 2020;21:6161). Once we have identified the cause, we can work on the necessary interventions.”
In a third study, machine-learning techniques demonstrated high predictive performance for the gut microbiome signature (GMS) among patients receiving immune checkpoint inhibitors across three centres in China (Abstract 45P). In a validation cohort, the GMS had a higher predictive value than traditional single gut microbiome biomarkers (p<0.05). SHapley Additive exPlanations analysis of the machine-learning model outputs identified the top 5 taxa driving GMS predictions as Anaerobutyricum hallii, Bacteroides eggerthii, Faecalibaculum rodentium, Clostridiales and Anaerostipes hadrus. In addition to its predictive capability, the GMS showed prognostic value for both OS and progression-free survival (log-rank p<0.001, in each case).
“Machine-learning techniques go a long way towards helping to identify species of interest and population trends, but what would be most useful in the clinic is a tool that guides personalised interventions,” says Zitvogel. Based on ecological topology of gut microbiota associated with immunotherapy resistance and response, a ‘TOPOSCORE’ has been created and translated into a qPCR-based assay (Cell. 2024;187:3373–3389). “The eventual aim is to develop a clinically actionable user-friendly tool to support the application of microbiome profiling of clinically significant intestinal dysbiosis in routine oncology practice,” she concludes.
Programme details
Hakozaki T, et al. Exploration of gut microbiota biomarkers in treatment-naïve advanced NSCLC patients undergoing chemo-immunotherapy: insights from the phase III trial, JCOG2007 (NIPPON). ESMO Immuno-Oncology Congress 2024, Abstract 1O
Proffered Paper Session, 11.12.2024, h. 16:15 – 18:00, Room B
Afzal Z, et al. Abnormal gut microbiota may cause PD-1 inhibitor-related cardiotoxicity via suppressing regulatory T cells. ESMO Immuno-Oncology Congress 2024, Abstract 46P
Poster Display Session, 12.12.2024, h. 12:30 – 13:30, Foyer mezzanine
Li H, et al. Gut microbiome signatures for exploring the correlation between gut microbiome and immune therapy response using machine learning approach. ESMO Immuno-Oncology Congress 2024, Abstract 45P
Poster Display Session, 12.12.2024, h. 12:30 – 13:30, Foyer mezzanine